BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons
- PMID: 22412021
- PMCID: PMC3308691
- DOI: 10.1083/jcb.201201038
BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons
Abstract
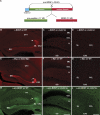
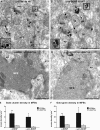
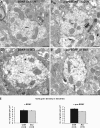
Although brain-derived neurotrophic factor (BDNF) regulates numerous and complex biological processes including memory retention, its extremely low levels in the mature central nervous system have greatly complicated attempts to reliably localize it. Using rigorous specificity controls, we found that antibodies reacting either with BDNF or its pro-peptide both stained large dense core vesicles in excitatory presynaptic terminals of the adult mouse hippocampus. Both moieties were ~10-fold more abundant than pro-BDNF. The lack of postsynaptic localization was confirmed in Bassoon mutants, a seizure-prone mouse line exhibiting markedly elevated levels of BDNF. These findings challenge previous conclusions based on work with cultured neurons, which suggested activity-dependent dendritic synthesis and release of BDNF. They instead provide an ultrastructural basis for an anterograde mode of action of BDNF, contrasting with the long-established retrograde model derived from experiments with nerve growth factor in the peripheral nervous system.
Figures







References
-
- Altrock W.D., tom Dieck S., Sokolov M., Meyer A.C., Sigler A., Brakebusch C., Fässler R., Richter K., Boeckers T.M., Potschka H., et al. 2003. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron. 37:787–800 10.1016/S0896-6273(03)00088-6 - DOI - PubMed
-
- Amaral, D., and P. Lavanex. 2006. Hippocampal Neuroanatomy. In The Hippocampus Book. P. Andersen, R. Morris, D. Amaral, T. Bliss, and J. O’Keefe, editors. Oxford University Press, Oxford. 37–114.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

