Structural insight into MR1-mediated recognition of the mucosal associated invariant T cell receptor
- PMID: 22412157
- PMCID: PMC3328369
- DOI: 10.1084/jem.20112095
Structural insight into MR1-mediated recognition of the mucosal associated invariant T cell receptor
Abstract
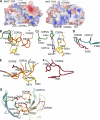
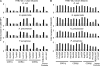
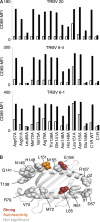
Mucosal-associated invariant T (MAIT) cells express a semiinvariant αβ T cell receptor (TCR) that binds MHC class I-like molecule (MR1). However, the molecular basis for MAIT TCR recognition by MR1 is unknown. In this study, we present the crystal structure of a human Vα7.2Jα33-Vβ2 MAIT TCR. Mutagenesis revealed highly conserved requirements for the MAIT TCR-MR1 interaction across different human MAIT TCRs stimulated by distinct microbial sources. Individual residues within the MAIT TCR β chain were dispensable for the interaction with MR1, whereas the invariant MAIT TCR α chain controlled specificity through a small number of residues, which are conserved across species and located within the Vα-Jα regions. Mutagenesis of MR1 showed that only two residues, which were centrally positioned and on opposing sides of the antigen-binding cleft of MR1, were essential for MAIT cell activation. The mutagenesis data are consistent with a centrally located MAIT TCR-MR1 docking that was dominated by the α chain of the MAIT TCR. This candidate docking mode contrasts with that of the NKT TCR-CD1d-antigen interaction, in which both the α and β chain of the NKT TCR is required for ligation above the F'-pocket of CD1d.
Figures

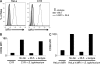


References
-
- Archbold J.K., Macdonald W.A., Gras S., Ely L.K., Miles J.J., Bell M.J., Brennan R.M., Beddoe T., Wilce M.C., Clements C.S., et al. 2009. Natural micropolymorphism in human leukocyte antigens provides a basis for genetic control of antigen recognition. J. Exp. Med. 206:209–219 10.1084/jem.20082136 - DOI - PMC - PubMed
-
- Borg N.A., Ely L.K., Beddoe T., Macdonald W.A., Reid H.H., Clements C.S., Purcell A.W., Kjer-Nielsen L., Miles J.J., Burrows S.R., et al. 2005. The CDR3 regions of an immunodominant T cell receptor dictate the ‘energetic landscape’ of peptide-MHC recognition. Nat. Immunol. 6:171–180 10.1038/ni1155 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

