Capsule type of Streptococcus pneumoniae determines growth phenotype
- PMID: 22412375
- PMCID: PMC3297593
- DOI: 10.1371/journal.ppat.1002574
Capsule type of Streptococcus pneumoniae determines growth phenotype
Abstract
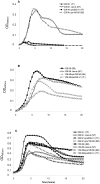
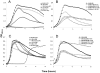
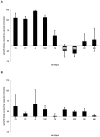
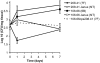
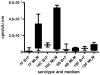
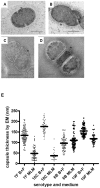
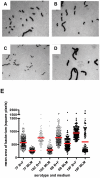
The polysaccharide capsule of Streptococcus pneumoniae defines over ninety serotypes, which differ in their carriage prevalence and invasiveness for poorly understood reasons. Recently, an inverse correlation between carriage prevalence and oligosaccharide structure of a given capsule has been described. Our previous work suggested a link between serotype and growth in vitro. Here we investigate whether capsule production interferes with growth in vitro and whether this predicts carriage prevalence in vivo. Eighty-one capsule switch mutants were constructed representing nine different serotypes, five of low (4, 7F, 14, 15, 18C) and four of high carriage prevalence (6B, 9V, 19F, 23F). Growth (length of lag phase, maximum optical density) of wildtype strains, nontypeable mutants and capsule switch mutants was studied in nutrient-restricted Lacks medium (MLM) and in rich undefined brain heart infusion broth supplemented with 5% foetal calf serum (BHI+FCS). In MLM growth phenotype depended on, and was transferred with, capsule operon type. Colonization efficiency of mouse nasopharynx also depended on, and was transferred with, capsule operon type. Capsule production interfered with growth, which correlated inversely with serotype-specific carriage prevalence. Serotypes with better growth and higher carriage prevalence produced thicker capsules (by electron microscopy, FITC-dextran exclusion assays and HPLC) than serotypes with delayed growth and low carriage prevalence. However, expression of cpsA, the first capsule gene, (by quantitative RT-PCR) correlated inversely with capsule thickness. Energy spent for capsule production (incorporation of H3-glucose) relative to amount of capsule produced was higher for serotypes with low carriage prevalence. Experiments in BHI+FCS showed overall better bacterial growth and more capsule production than growth in MLM and differences between serotypes were no longer apparent. Production of polysaccharide capsule in S. pneumoniae interferes with growth in nutrient-limiting conditions probably by competition for energy against the central metabolism. Serotype-specific nasopharyngeal carriage prevalence in vivo is predicted by the growth phenotype.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Jin P, Kong F, Xiao M, Oftadeh S, Zhou F, et al. First report of putative Streptococcus pneumoniae serotype 6D among nasopharyngeal isolates from Fijian children. J Infect Dis. 2009;200:1375–1380. - PubMed
-
- Moxon E, Kroll J. The role of bacterial polysaccharide capsules as virulence factors. Curr Top Microbiol Immunol. 1990;150:65–85. - PubMed
-
- Watson D, Musher D. A brief history of the pneumococcus in biomedical research. Semin Respir Infect. 1999;14:198–208. - PubMed
-
- Brueggemann A, Peto T, Crook D, Butler J, Kristinsson K, et al. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis. 2004;190:1203–1211. - PubMed

