Tris(3-nitro-pentane-2,4-dionato-κO,O')-cobalt(III)
- PMID: 22412453
- PMCID: PMC3297263
- DOI: 10.1107/S160053681200668X
Tris(3-nitro-pentane-2,4-dionato-κO,O')-cobalt(III)
Abstract
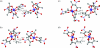
The structure of the title compound, [Co(C(5)H(6)NO(4))(3)], consists of a Co(III) ion octahedrally coordinated by three bidentate 3-nitro-pentane-2,4-dionate ligands. The complex was prepared via the nitration of tris-(2,4-penta-nedionato-κ(2)O,O')cobalt(III) with a solution of copper(II) nitrate in glacial acetic acid. The central C atom and the nitro group of one 3-nitro-pentane-2,4-dionate ligand are disordered over two positions with an occupancy ratio of 0.848 (4):0.152 (4). A second nitro group is also disordered over two orientations with an occupancy ratio of 0.892 (7):0.108 (7). Two of the ligand methyl groups form C-H⋯O inter-actions with two different nitro groups to form chains running along the c axis. Additional C-H⋯O inter-actions are found between ligand methyl groups and the cobalt-bound O atoms, also resulting in the formation of chains along the c axis.
Figures



References
-
- Abrahams, B. F., Hoskins, B. F., McFadyen, D. W. & Perrin, L. C. (1998). Acta Cryst. C54, 1807–1809.
-
- Appleton, T. G., Gahan, L. R. & Oliver, P. J. (1992). Aust. J. Chem. 45, 797–805.
-
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N. L. (1995). Angew. Chem. Int. Ed. 34, 1555–1573.
-
- Bruker (2009). GIS, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cason, C. J. (2004). POV-RAY Persistence of Vision Raytracer Pty Ltd, Williamstown, Victoria, Australia.
LinkOut - more resources
Full Text Sources
