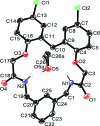
10,16-Dichloro-6,20-dioxa-3,23-diaza-tetra-cyclo-[23.3.1.0.0]nona-cosa-1(29),7,9,11,14(19),15,17,25,27-nona-ene-4,22-dione methanol monosolvate
- PMID: 22412673
- PMCID: PMC3297870
- DOI: 10.1107/S1600536812007052
10,16-Dichloro-6,20-dioxa-3,23-diaza-tetra-cyclo-[23.3.1.0.0]nona-cosa-1(29),7,9,11,14(19),15,17,25,27-nona-ene-4,22-dione methanol monosolvate
Abstract
In the title compound, C(25)H(22)Cl(2)N(2)O(4)·CH(3)OH, the macrocyclic mol-ecule adopts a slightly distorted C(2)-symmetric conformation. The macrocyclic mol-ecules are linked via N-H⋯O hydrogen bonds between the amide groups into chains extending along the [010] direction. The methanol mol-ecules bridge these chains via N-H⋯O and O-H⋯O hydrogen bonds with the formation of a two-dimensional polymeric structure parallel to (001). The methanol mol-ecule is disordered over two positions with the occupancy ratio of 9:1. The disorder of the solvent molecule is caused by weak intermolecular C-H⋯Cl hydrogen bonding.
Figures

References
-
- Agilent (2010). CrysAlis PRO. Agilent Technologies, Yarnton, Oxfordshire, England.
-
- Alexander, V. (1995). Chem. Rev. 95, 273–342.
-
- Christensen, A., Jensen, H. S., McKee, V., Mckenzie, C. J. & Munch, M. (1997). Inorg. Chem. 36, 6080–6085. - PubMed
-
- Ertul, S., Tombak, A. H., Bayrakci, M. & Merter, O. (2009). Acta Chim. Slov. 56, 878–884.
-
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
LinkOut - more resources
Full Text Sources
