Prolyl isomerase Pin1 regulates mouse embryonic fibroblast differentiation into adipose cells
- PMID: 22412843
- PMCID: PMC3296696
- DOI: 10.1371/journal.pone.0031823
Prolyl isomerase Pin1 regulates mouse embryonic fibroblast differentiation into adipose cells
Abstract
Background: A peptidyl prolyl cis/trans isomerase, Pin1, regulates insulin signal transduction. Pin1 reduces responses to insulin stimulation by binding CRTC2 (CREB-regulated transcriptional co-activator 2) and PPARγ (peroxisome prolifereator- activated receptor γ), but conversely enhances insulin signaling by binding IRS-1 (insulin receptor substrate-1), Akt kinase, and Smad3. Therefore, it is still unclear whether Pin1 inhibits or enhances adipose cell differentiation.
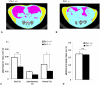
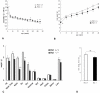
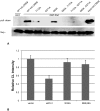
Methodology/principal findings: Pin1(-/-) and wild-type mice were fed with high fat diets and adipose tissue weight was measured. Compared to wild-type mice, Pin1(-/-) mice had lower adipose tissue weight, while the weight of other tissues was similar. Mouse embryo fibroblasts (MEFs), prepared from both groups of mice, were induced to differentiate into adipose cells by stimulation with insulin. However, the rate of differentiation of MEFs from Pin1(-/-) mice was less than that of MEFs from wild-type mice. The rate of insulin-induced MEF cell differentiation in Pin1(-/-) mice was restored by increasing expression of Pin1. We found that Pin1 binds to phosphoThr172- and phosphoSer271-Pro sites in CREB suppress the activity in COS-7 cells.
Conclusion and significance: Pin1 enhanced the uptake of triglycerides and the differentiation of MEF cells into adipose cells in response to insulin stimulation. Results of this study suggest that Pin1 down-regulation could be a potential approach in obesity-related dysfunctions, such as high blood pressure, diabetes, non-alcoholic steatohepatitis.
Conflict of interest statement
Figures


References
-
- Fujimori F, Takahashi K, Uchida C, Uchida T. Mice lacking Pin1 develop normally, but are defective in entering cell cycle from G0 arrest. Biochem Biophys Res Commun. 1999;265:658–663. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

