The safety, effectiveness and concentrations of adjusted lopinavir/ritonavir in HIV-infected adults on rifampicin-based antitubercular therapy
- PMID: 22412856
- PMCID: PMC3296695
- DOI: 10.1371/journal.pone.0032173
The safety, effectiveness and concentrations of adjusted lopinavir/ritonavir in HIV-infected adults on rifampicin-based antitubercular therapy
Abstract
Objective: Rifampicin co-administration dramatically reduces plasma lopinavir concentrations. Studies in healthy volunteers and HIV-infected patients showed that doubling the dose of lopinavir/ritonavir (LPV/r) or adding additional ritonavir offsets this interaction. However, high rates of hepatotoxicity were observed in healthy volunteers. We evaluated the safety, effectiveness and pre-dose concentrations of adjusted doses of LPV/r in HIV infected adults treated with rifampicin-based tuberculosis treatment.
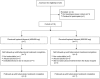
Methods: Adult patients on a LPV/r-based antiretroviral regimen and rifampicin-based tuberculosis therapy were enrolled. Doubled doses of LPV/r or an additional 300 mg of ritonavir were used to overcome the inducing effect of rifampicin. Steady-state lopinavir pre-dose concentrations were evaluated every second month.
Results: 18 patients were enrolled with a total of 79 patient months of observation. 11/18 patients were followed up until tuberculosis treatment completion. During tuberculosis treatment, the median (IQR) pre-dose lopinavir concentration was 6.8 (1.1-9.2) mg/L and 36/47 (77%) were above the recommended trough concentration of 1 mg/L. Treatment was generally well tolerated with no grade 3 or 4 toxicity: 8 patients developed grade 1 or 2 transaminase elevation, 1 patient defaulted additional ritonavir due to nausea and 1 patient developed diarrhea requiring dose reduction. Viral loads after tuberculosis treatment were available for 11 patients and 10 were undetectable.
Conclusion: Once established on treatment, adjusted doses of LPV/r co-administered with rifampicin-based tuberculosis treatment were tolerated and LPV pre-dose concentrations were adequate.
Conflict of interest statement
Figures

References
-
- CDC. Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis. 2007. Available: http://www.cdc.gov/tb/publications/guidelines/HIV_AIDS.htm. Accessed 2010 Aug 13.
-
- Unexpected hepatotoxicity observed in a healthy volunteer study on the effects of multiple dose rifampicin on the steady-state pharmacokinetics of ritonavir-boosted sequinavir and vice versa. 2005. Sixth International Workshop on Clinical Pharmacology of HIV therapy; 28–30 April; Montreal, Quebec, Canada.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

