Evidence that Ca2+ within the microdomain of the L-type voltage gated Ca2+ channel activates ERK in MIN6 cells in response to glucagon-like peptide-1
- PMID: 22412973
- PMCID: PMC3296766
- DOI: 10.1371/journal.pone.0033004
Evidence that Ca2+ within the microdomain of the L-type voltage gated Ca2+ channel activates ERK in MIN6 cells in response to glucagon-like peptide-1
Abstract
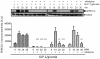
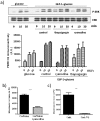
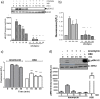
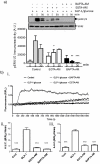
Glucagon like peptide-1 (GLP-1) is released from intestinal L-cells in response to nutrient ingestion and acts upon pancreatic β-cells potentiating glucose-stimulated insulin secretion and stimulating β-cell proliferation, differentiation, survival and gene transcription. These effects are mediated through the activation of multiple signal transduction pathways including the extracellular regulated kinase (ERK) pathway. We have previously reported that GLP-1 activates ERK through a mechanism dependent upon the influx of extracellular Ca(2+) through L-type voltage gated Ca(2+) channels (VGCC). However, the mechanism by which L-type VGCCs couple to the ERK signalling pathway in pancreatic β-cells is poorly understood. In this report, we characterise the relationship between L-type VGCC mediated changes in intracellular Ca(2+) concentration ([Ca(2+)](i)) and the activation of ERK, and demonstrate that the sustained activation of ERK (up to 30 min) in response to GLP-1 requires the continual activation of the L-type VGCC yet does not require a sustained increase in global [Ca(2+)](i) or Ca(2+) efflux from the endoplasmic reticulum. Moreover, sustained elevation of [Ca(2+)](i) induced by ionomycin is insufficient to stimulate the prolonged activation of ERK. Using the cell permeant Ca(2+) chelators, EGTA-AM and BAPTA-AM, to determine the spatial dynamics of L-type VGCC-dependent Ca(2+) signalling to ERK, we provide evidence that a sustained increase in Ca(2+) within the microdomain of the L-type VGCC is sufficient for signalling to ERK and that this plays an important role in GLP-1- stimulated ERK activation.
Conflict of interest statement
Figures


References
-
- Perfetti R, Merkel P. Glucagon-like peptide-1: a major regulator of pancreatic beta-cell function. Eur J Endocrinol. 2000;143:717–725. - PubMed
-
- Arnette D, Gibson TB, Lawrence MC, January B, Khoo S, et al. Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic beta cells. J Biol Chem. 2003;278:32517–32525. - PubMed
-
- Gomez E, Pritchard C, Herbert TP. cAMP-dependent protein kinase and Ca2+ influx through L-type voltage-gated calcium channels mediate Raf-independent activation of extracellular regulated kinase in response to glucagon-like peptide-1 in pancreatic beta-cells. J Biol Chem. 2002;277:48146–48151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

