A disintegrin and metalloenzyme (ADAM) 17 activation is regulated by α5β1 integrin in kidney mesangial cells
- PMID: 22413019
- PMCID: PMC3297637
- DOI: 10.1371/journal.pone.0033350
A disintegrin and metalloenzyme (ADAM) 17 activation is regulated by α5β1 integrin in kidney mesangial cells
Abstract
Background: The disintegrin and metalloenzyme ADAM17 participates in numerous inflammatory and proliferative diseases, and its pathophysiological role was implicated in kidney fibrosis, polycystic kidney disease and other chronic kidney diseases. At present, we have little understanding how the enzyme activity is regulated. In this study we wanted to characterize the role of α5β1 integrin in ADAM17 activity regulation during G protein-coupled receptor (GPCR) stimulation.
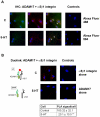
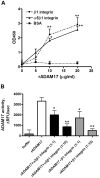
Methodology/principal findings: We showed previously that the profibrotic GPCR agonist serotonin (5-HT) induced kidney mesangial cell proliferation through ADAM17 activation and heparin-binding epidermal growth factor (HB-EGF) shedding. In the present studies we observed that in unstimulated mesangial cell lysates α5β1 integrin co-precipitated with ADAM17 and that 5-HT treatment of the cells induced dissociation of α5β1 integrin from ADAM17. Using fluorescence immunostaining and in situ proximity ligation assay, we identified the perinuclear region as the localization of the ADAM17/α5β1 integrin interaction. In cell-free assays, we showed that purified α5β1 integrin and β1 integrin dose-dependently bound to and inhibited activity of recombinant ADAM17. We provided evidence that the conformation of the integrin determines its ADAM17-binding ability. To study the effect of β1 integrin on ADAM17 sheddase activity, we employed alkaline phosphatase-tagged HB-EGF. Overexpression of β1 integrin lead to complete inhibition of 5-HT-induced HB-EGF shedding and silencing β1 integrin by siRNA significantly increased mesangial cells ADAM17 responsiveness to 5-HT.
Conclusions/significance: Our data show for the first time that β1 integrin has an important physiological role in ADAM17 activity regulation. We suggest that regulating α5β1 integrin binding to ADAM17 could be an attractive therapeutic target in chronic kidney diseases.
Conflict of interest statement
Figures



Similar articles
-
5-HT2A receptor induces ERK phosphorylation and proliferation through ADAM-17 tumor necrosis factor-alpha-converting enzyme (TACE) activation and heparin-bound epidermal growth factor-like growth factor (HB-EGF) shedding in mesangial cells.J Biol Chem. 2006 Jul 28;281(30):21004-21012. doi: 10.1074/jbc.M512096200. Epub 2006 May 31. J Biol Chem. 2006. PMID: 16737974
-
HB-EGF release mediates glucose-induced activation of the epidermal growth factor receptor in mesangial cells.Am J Physiol Renal Physiol. 2011 Apr;300(4):F921-31. doi: 10.1152/ajprenal.00436.2010. Epub 2011 Feb 2. Am J Physiol Renal Physiol. 2011. PMID: 21289053
-
Helicobacter pylori CagL activates ADAM17 to induce repression of the gastric H, K-ATPase alpha subunit.Gastroenterology. 2010 Jul;139(1):239-48. doi: 10.1053/j.gastro.2010.03.036. Epub 2010 Mar 18. Gastroenterology. 2010. PMID: 20303353 Free PMC article.
-
TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase.Ann N Y Acad Sci. 2003 May;995:22-38. doi: 10.1111/j.1749-6632.2003.tb03207.x. Ann N Y Acad Sci. 2003. PMID: 12814936 Review.
-
ADAM-mediated ectodomain shedding of HB-EGF in receptor cross-talk.Biochim Biophys Acta. 2005 Aug 1;1751(1):110-7. doi: 10.1016/j.bbapap.2004.11.009. Epub 2004 Dec 8. Biochim Biophys Acta. 2005. PMID: 16054021 Review.
Cited by
-
Novel Markers in Diabetic Kidney Disease-Current State and Perspectives.Diagnostics (Basel). 2022 May 11;12(5):1205. doi: 10.3390/diagnostics12051205. Diagnostics (Basel). 2022. PMID: 35626360 Free PMC article. Review.
-
Cellular Integrin α5β1 and Exosomal ADAM17 Mediate the Binding and Uptake of Exosomes Produced by Colorectal Carcinoma Cells.Int J Mol Sci. 2021 Sep 14;22(18):9938. doi: 10.3390/ijms22189938. Int J Mol Sci. 2021. PMID: 34576100 Free PMC article.
-
ADAM17 and EGFR regulate IL-6 receptor and amphiregulin mRNA expression and release in cigarette smoke-exposed primary bronchial epithelial cells from patients with chronic obstructive pulmonary disease (COPD).Physiol Rep. 2016 Aug;4(16):e12878. doi: 10.14814/phy2.12878. Physiol Rep. 2016. PMID: 27561911 Free PMC article.
-
ADAM17 is a survival factor for microglial cells in vitro and in vivo after spinal cord injury in mice.Cell Death Dis. 2013 Dec 12;4(12):e954. doi: 10.1038/cddis.2013.466. Cell Death Dis. 2013. PMID: 24336074 Free PMC article.
-
A disintegrin and metalloprotease 17 dynamic interaction sequence, the sweet tooth for the human interleukin 6 receptor.J Biol Chem. 2014 Jun 6;289(23):16336-48. doi: 10.1074/jbc.M114.557322. Epub 2014 Apr 30. J Biol Chem. 2014. PMID: 24790088 Free PMC article.
References
-
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. - PubMed
-
- Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–736. - PubMed
-
- Horiuchi K, Zhou HM, Kelly K, Manova K, Blobel CP. Evaluation of the contributions of ADAMs 9, 12, 15, 17, and 19 to heart development and ectodomain shedding of neuregulins beta1 and beta2. Dev Biol. 2005;283:459–471. - PubMed
-
- Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. - PubMed
-
- Buckley CA, Rouhani FN, Kaler M, Adamik B, Hawari FI, et al. Amino-terminal TACE prodomain attenuates TNFR2 cleavage independently of the cysteine switch. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1132–1138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

