Public support for neonatal screening for Pompe disease, a broad-phenotype condition
- PMID: 22413814
- PMCID: PMC3351372
- DOI: 10.1186/1750-1172-7-15
Public support for neonatal screening for Pompe disease, a broad-phenotype condition
Abstract
Background: Neonatal screening for Pompe disease has been introduced in Taiwan and a few U.S. states, while other jurisdictions including some European countries are piloting or considering this screening. First-tier screening flags both classic infantile and late-onset Pompe disease, which challenges current screening criteria. Previously, advocacy groups have sometimes supported expanded neonatal screening more than professional experts, while neutral citizens' views were unknown. This study aimed to measure support for neonatal screening for Pompe disease in the general public and to compare it to support among (parents of) patients with this condition. The study was done in the Netherlands, where newborns are not currently screened for Pompe disease. Newborn screening is not mandatory in the Netherlands but current uptake is almost universal.
Methods: A consumer panel (neutral group) and (parents of) patients with Pompe disease (Pompe group) were sent information and a questionnaire. Responses were analyzed of 555 neutral and 58 Pompe-experienced informants who had demonstrated sufficient understanding.
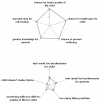
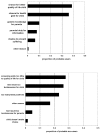
Results: 87% of the neutral group and 88% of the Pompe group supported the introduction of screening (95% CI of difference -10 to 7%). The groups were similar in their moral reasoning about screening and acceptance of false positives, but the Pompe-experienced group expected greater benefit from neonatal detection of late-onset disease. Multivariate regression analysis controlling for demographics confirmed that approval of the introduction of screening was independent of having (a child with) Pompe disease. Furthermore, respondents with university education, regardless of whether they have (a child with) Pompe disease, were more likely to be reluctant about the introduction of screening than those with less education, OR for approval 0.29 (95% CI 0.18 to 0.49, p < 0.001).
Conclusions: This survey suggests a rather high level of support for newborn screening for Pompe disease, not only among those who have personal experience of the disease but also among the general public in the Netherlands. Optional screening on the basis of informed parental consent is probably unrealistic, underlining the need for new guidelines to help policymakers in their consideration of newborn screening for broad phenotype conditions.
Figures
References
-
- ISNS General Guidelines for Neonatal Screening. International Society for Neonatal Screening. 2008. http://www.isns-neoscreening.org/htm/generalguidelines.htm [Accessed Nov.2, 2011]
-
- The President's Council on Bioethics. The changing moral focus of newborn screening. 2008. http://bioethics.georgetown.edu/pcbe/reports/newborn_screening/Newborn%2... [Accessed Nov.2, 2011]
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

