Double-fold C-H oxygenation of arenes using PyrDipSi: a general and efficient traceless/modifiable silicon-tethered directing group
- PMID: 22414133
- PMCID: PMC3371766
- DOI: 10.1021/ja3010545
Double-fold C-H oxygenation of arenes using PyrDipSi: a general and efficient traceless/modifiable silicon-tethered directing group
Abstract
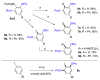
The efficient Pd-catalyzed double-fold C-H oxygenation of arenes into resorcinols using the newly developed 2-pyrimidyldiisopropylsilyl (PyrDipSi) directing group is described. Its use allows for the sequential introduction of OAc and OPiv groups in a one-pot manner to produce orthogonally protected resorcinol derivatives. The PyrDipSi group is superior to the previously developed 2-pyridyldiisopropylsilyl (PyDipSi) group, as it is efficient for monooxygenation of ortho-substituted arenes. Notably, the PyrDipSi group can be easily installed into arene molecules and can be easily removed or modified after the oxygenation reaction.
Figures




References
-
-
For selected general reviews on transition metal-catalyzed C-H activation, see: Wencel-Delord J, Dröge T, Liu F, Glorius F. Chem. Soc. Rev. 2011;40:4740. Wendlandt AE, Suess AM, Stahl SS. Angew. Chem., Int. Ed. 2011;50:11062. Sun C-L, Li B-J, Shi Z-J. Chem. Rev. 2011;111:1293. Yoshikai N. Synlett. 2011:1047. Ackermann L. Chem. Rev. 2011;111:1315. Patureau FW, Glorius F. Angew. Chem., Int. Ed. 2011;50:1977. Li H, Li B-J, Shi Z-J. Cat. Sci. Tech. 2011;1:191. Herrmann P, Bach T. Chem. Soc. Rev. 2011;40:2022. Engle KM, Mei T-S, Wasa M, Yu J-Q. Acc. Chem. Res. 2012 doi: 10.1021/ar200185g. Yeung CS, Dong VM. Chem. Rev. 2011;111:1215. Yu J-Q, Shi Z. In: Top. Cur. Chem. Yu J-Q, Shi Z, editors. Vol. 292. Springer; Heidelberg, Dordrecht, London, New York: 2010. Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. Sehnal P, Taylor RJK, Fairlamb IJS. Chem. Rev. 2010;110:824. Shul’pin GB. Org. Biomol. Chem. 2010;8:4217. Balcells D, Clot E, Eisenstein O. Chem. Rev. 2010;110:749. Giri R, Shi B-F, Engle KM, Maugel N, Yu J-Q. Chem. Soc. Rev. 2009;38:3242. Muñiz K. Angew. Chem., Int. Ed. 2009;48:9412. Seregin IV, Gevorgyan V. Chem. Soc. Rev. 2007;36:1173. Daugulis O, Zaitsev V, Shabashov D, Pham Q-N, Lazareva A. Synlett. 2006:3382. Labinger JA, Bercaw JE. Nature. 2002;417:507. Shilov AE, Shul’pin GB. Chem. Rev. 1997;97:2879.
-
-
-
For recent reviews on formation of C-C bond via transition metal-catalyzed C-H activation, see: Ackermann L. Angew. Chem., Int. Ed. 2011;50:3842. Bugaut X, Glorius F. Angew. Chem., Int. Ed. 2011;50:7479. Cho SH, Kim JY, Kwak J, Chang S. Chem. Soc. Rev. 2011;40:5068. You S-L, Xia J-B. Top. Curr. Chem. 2010;292:165. Satoh T, Miura M. Chem.–Eur. J. 2010;16:11212. Miura M, Satoh T. Synthesis. 2010:3395. Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624. Ashenhurst JA. Chem. Soc. Rev. 2010;39:540. McGlacken GP, Bateman LM. Chem. Soc. Rev. 2009;38:2447. Daugulis O, Do H-Q, Shabashov D. Acc. Chem. Res. 2009;42:1074. Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem., Int. Ed. 2009;48:5094. Bellina F, Rossi R. Tetrahedron. 2009;65:10269. Ackermann L, Vicente RN, Kapdi AR. Angew. Chem., Int. Ed. 2009;48:9792. Kulkarni AA, Daugulis O. Synthesis. 2009:4087. Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174.
-
-
-
For recent reviews on formation of C-heteroatom bond via transition metal-catalyzed C-H activation, see: Enthaler S, Company A. Chem. Soc. Rev. 2011;40:4912. Beccalli EM, Broggini G, Fasana A, Rigamonti MJ. Organomet. Chem. 2011;696:277. Zhang M, Zhang A. Synthesis. 2011;44:1. Stokes BJ, Driver TG. Eur. J. Org. Chem. 2011:4071. Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem. Rev. 2010;110:890. Collet F, Dodd RH, Dauban P. Chem. Commun. 2009:5061. Beccalli EM, Broggini G, Martinelli M, Sottocornola S. Chem. Rev. 2007;107:5318. Yu J-Q, Giri R, Chen X. Org. Biomol. Chem. 2006;4:4041.
-
-
-
For recent review on removable directing groups, see: Rousseau G, Breit B. Angew. Chem., Int. Ed. 2011;50:2450.
-
-
-
For recent applications of removable directing groups for transition metal-catalyzed C-H activation, see: Bedford RB, Coles SJ, Hursthouse MB, Limmert ME. Angew. Chem., Int. Ed. 2003;42:112. Oi S, Watanabe S.-i., Fukita S, Inoue Y. Tetrahedron Lett. 2003;44:8665. Bedford RB, Limmert ME. J. Org. Chem. 2003;68:8669. Bedford RB, Betham M, Caffyn AJM, Charmant JPH, Lewis-Alleyne LC, Long PD, Polo-Cerón D, Prashar S. Chem. Commun. 2008:990. Maehara A, Tsurugi H, Satoh T, Miura M. Org. Lett. 2008;10:1159. Boebel TA, Hartwig JF. J. Am. Chem. Soc. 2008;130:7534. Ihara H, Suginome M. J. Am. Chem. Soc. 2009;131:7502. García-Rubia A, Urones B, Gómez Arrayás R, Carretero JC. Chem.–Eur. J. 2010;16:9676. Robbins DW, Boebel TA, Hartwig JF. J. Am. Chem. Soc. 2010;132:4068. García-Rubia A, Fernández-Ibáñez MÁ, Gómez Arrayás R, Carretero JC. Chem.–Eur. J. 2011;17:3567. García-Rubia A, Fernández-Ibáñez MÁ, Gómez Arrayás R, Carretero JC. Angew. Chem., Int. Ed. 2011;50:10927. Huang C, Chattopadhyay B, Gevorgyan V. J. Am. Chem. Soc. 2011;133:12406. Ihara H, Ueda A, Suginome M. Chem. Lett. 2011;40:916. Richter H, Beckendorf S, Mancheño OG. Adv. Synth. Catal. 2011;353:295. Wang C, Ge H. Chem.–Eur. J. 2011;17:14371. Dai H-X, Stepan AF, Plummer MS, Zhang Y-H, Yu J-Q. J. Am. Chem. Soc. 2011;133:7222. Ackermann L, Diers E, Manvar A. Org. Lett. 2012;14:1154. Zhang X, Yu M, Yao J, Zhang Y. Synlett. 2012:463.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

