Decreased cocaine motor sensitization and self-administration in mice overexpressing cannabinoid CB₂ receptors
- PMID: 22414816
- PMCID: PMC3358745
- DOI: 10.1038/npp.2012.22
Decreased cocaine motor sensitization and self-administration in mice overexpressing cannabinoid CB₂ receptors
Abstract
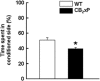
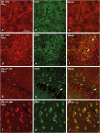
The potential involvement of the cannabinoid CB₂ receptors (CB₂r) in the adaptive responses induced by cocaine was studied in transgenic mice overexpressing the CB₂r (CB₂xP) and in wild-type (WT) littermates. For this purpose, the acute and sensitized locomotor responses to cocaine, conditioned place preference, and cocaine intravenous self-administration were evaluated. In addition, we assessed whether CB₂r were localized in neurons and/or astrocytes, and whether they colocalized with dopamine D1 and D2 receptors (D1Dr and D2Dr). Dopamine (DA) extracellular levels in the nucleus accumbens (NAcc), and gene expression of tyrosine hydroxylase (TH) and DA transporter (DAT) in the ventral tegmental area (VTA), and μ-opioid and cannabinoid CB₁ receptors in the NAcc were also studied in both genotypes. CB₂xP mice showed decreased motor response to acute administration of cocaine (10-20 mg/kg) and cocaine-induced motor sensitization compared with WT mice. CB₂xP mice presented cocaine-induced conditioned place aversion and self-administered less cocaine than WT mice. CB₂r were found in neurons and astrocytes and colocalized with D2Dr in the VTA and NAcc. No significant differences in extracellular DA levels in the NAcc were observed between genotypes after cocaine administration. Under baseline conditions, TH and DAT gene expression was higher and μ-opioid receptor gene expression was lower in CB₂xP than in WT mice. However, both genotypes showed similar changes in TH and μ-opioid receptor gene expression after cocaine challenge independently of the pretreatment received. Importantly, the cocaine challenge decreased DAT gene expression to a lesser extent in cocaine-pretreated CB₂xP than in cocaine-pretreated WT mice. These results revealed that CB₂r are involved in cocaine motor responses and cocaine self-administration, suggesting that this receptor could represent a promising target to develop novel treatments for cocaine addiction.
Figures
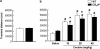


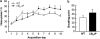


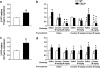

References
-
- Andre VM, Cepeda C, Cummings DM, Jocoy EL, Fisher YE, William Yang X, et al. Dopamine modulation of excitatory currents in the striatum is dictated by the expression of D1 or D2 receptors and modified by endocannabinoids. Eur J Neurosci. 2010;31:14–28. - PubMed
-
- Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M, et al. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65. - PubMed
-
- Chefer VI, Kieffer BL, Shippenberg TS. Contrasting effects of mu opioid receptor and delta opioid receptor deletion upon the behavioral and neurochemical effects of cocaine. Neuroscience. 2004;127:497–503. - PubMed
-
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, et al. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–1154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

