Spleen cells from young but not old immunized mice eradicate large established cancers
- PMID: 22415314
- PMCID: PMC5354938
- DOI: 10.1158/1078-0432.CCR-12-0127
Spleen cells from young but not old immunized mice eradicate large established cancers
Abstract
Purpose: Solid tumors that have grown two weeks or longer in mice and have diameters larger than 1 cm are histologically indistinguishable from autochthonous human cancers. When experimental tumors reach this clinically relevant size, they are usually refractory to most immunotherapies but may be destroyed by adoptive T-cell transfer. However, TCR-transgenic T cells and/or tumor cells overexpressing antigens are frequently used in these experiments. Here we studied the requirements for destroying clinical size, unmanipulated 8101 tumors by adoptive cell therapy.
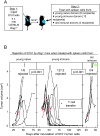
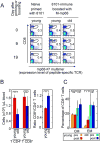
Experimental design: 8101 arose in an old mouse after chronic exposure to UV light. A cancer line was established, which was never serially transplanted. The immunodominant CD8(+) T cell-recognized antigen of this tumor is caused by a somatic tumor-specific mutation in the RNA helicase p68. 8101 tumors were treated with spleen cells from young naive, or young and old immunized mice to ascertain the characteristics of immune cells that lead to rejection.
Results: Here we show that the mutant p68 peptide has an exceptionally high affinity to the presenting MHC class I molecule K(b) and that spleen cells from immunized young syngeneic mice adoptively transferred to Rag(-/-) or cancer-suppressed euthymic mice eradicate 8101 tumors larger than 1 cm in average diameter and established for several weeks. Spleen cells from naive young mice or from old and boosted (reimmunized) mice were ineffective.
Conclusions: Relapse-free destruction of large and long-established tumors expressing a genuine very high-affinity tumor-specific antigen can be achieved by using adoptive transfer of lymphocytes from immunized young individuals.
©2012 AACR.
Figures


Comment in
-
Spleen cells from young donors eradicate large tumors--letter.Clin Cancer Res. 2012 Nov 1;18(21):6074-5; author reply 6076. doi: 10.1158/1078-0432.CCR-12-2369. Epub 2012 Oct 15. Clin Cancer Res. 2012. PMID: 23071259 No abstract available.
References
-
- Stricker TP, Kumar V. Neoplasia. In: Kumar V, Abbas AK, Fausto N, Aster J, editors. Robbins & Cotran Pathologic Basis of Disease. 8. Philadelphia: WB Saunders; 2009.
-
- Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10:294–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

