Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis
- PMID: 22415826
- PMCID: PMC3316881
- DOI: 10.1038/ncomms1734
Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis
Abstract
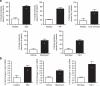
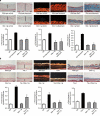
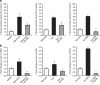
The transforming growth factor-β (TGF-β) signalling pathway is a key mediator of fibroblast activation that drives the aberrant synthesis of extracellular matrix in fibrotic diseases. Here we demonstrate a novel link between transforming growth factor-β and the canonical Wnt pathway. TGF-β stimulates canonical Wnt signalling in a p38-dependent manner by decreasing the expression of the Wnt antagonist Dickkopf-1. Tissue samples from human fibrotic diseases show enhanced expression of Wnt proteins and decreased expression of Dickkopf-1. Activation of the canonical Wnt pathway stimulates fibroblasts in vitro and induces fibrosis in vivo. Transgenic overexpression of Dickkopf-1 ameliorates skin fibrosis induced by constitutively active TGF-β receptor type I signalling and also prevents fibrosis in other TGF-β-dependent animal models. These findings demonstrate that canonical Wnt signalling is necessary for TGF-β-mediated fibrosis and highlight a key role for the interaction of both pathways in the pathogenesis of fibrotic diseases.
Figures


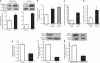


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

