Fast and ultrasensitive method for quantitating prion infectivity titre
- PMID: 22415832
- PMCID: PMC3518416
- DOI: 10.1038/ncomms1730
Fast and ultrasensitive method for quantitating prion infectivity titre
Abstract
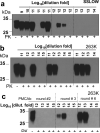
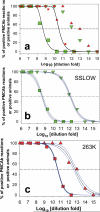
Bioassay by end-point dilution has been used for decades for routine determination of prion infectivity titre. Here we show that the new protein misfolding cyclic amplification with beads (PMCAb) technique can be used to estimate titres of the infection-specific forms of the prion protein with a higher level of precision and in 3-6 days as opposed to 2 years, when compared with the bioassay. For two hamster strains, 263 K and SSLOW, the median reactive doses determined by PCMAb (PMCAb(50)) were found to be 10(12.8) and 10(12.2) per gram of brain tissue, which are 160- and 4,000-fold higher than the corresponding median infectious dose (ID(50)) values measured by bioassay. The 10(2)- to 10(3)-fold differences between ID(50) and PMCAb(50) values could be due to a large excess of PMCAb-reactive prion protein seeds with little or no infectivity. Alternatively, the differences between ID(50) and PMCAb(50) could be due to higher rate of clearance of infection-specific prion protein seeds in animals versus PMCAb reactions. A well-calibrated PMCAb reaction can be an efficient and cost-effective method for the estimation of infection-specific prion protein titre.
Figures

References
-
- Wadsworth JD, et al. Tissues distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunobloting assay. Lancet. 2001;358:171–180. - PubMed
-
- Safar JG, et al. Measuring prions causing bovine spongiform encephalopathy or chronic wasting disease by immunoassays and transgenic mice. Nat. Biotechnol. 2002;20:1147–1150. - PubMed
-
- Edgeworth JA, et al. Detection of prion infection in variant Creutzfeldt-Jakob disease: a blood-based assay. Lancet. 2011;377:487–493. - PubMed
-
- Gregori L, et al. A sensitive and quantitative assay for normal PrP in plasma. J. Virol Methods. 2008;149:251–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

