The AGE-breaker ALT-711 restores high blood flow-dependent remodeling in mesenteric resistance arteries in a rat model of type 2 diabetes
- PMID: 22415880
- PMCID: PMC3357287
- DOI: 10.2337/db11-0750
The AGE-breaker ALT-711 restores high blood flow-dependent remodeling in mesenteric resistance arteries in a rat model of type 2 diabetes
Abstract
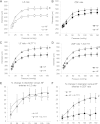
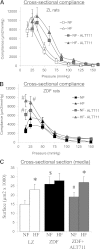
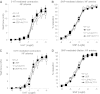
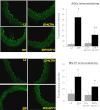
Flow-mediated remodeling of resistance arteries is essential for revascularization in ischemic diseases, but this is impaired in diabetes. We hypothesized that breaking advanced glycation end product (AGE) cross-links could improve remodeling in mesenteric resistance arteries in Zucker diabetic fatty (ZDF) rats compared with lean Zucker (LZ) rats. Arteries, exposed to high (HF) or normal (NF) blood flow after alternate arterial ligation in vivo, were collected after 2 weeks. In LZ rats, HF artery diameter was larger than for NF vessels, but this was not the case in ZDF rats. Endothelium-mediated dilation in ZDF rats, which was lower than in LZ rats, was further decreased in HF arteries. Treatment of rats with the AGE-breaker 4,5-dimethyl-3-phenacylthiazolium chloride (ALT-711) (3 mg/kg/day; 3 weeks) reversed diabetes-induced impairment of HF-dependent remodeling. ALT-711 also improved endothelium nitric oxide-dependent relaxation in mesenteric resistance arteries. Reactive oxygen species reduction restored relaxation in ZDF rats but not in LZ or ALT-711-treated rats. AGEs were reduced in ALT-711-treated ZDF rats compared with ZDF rats. Metalloproteinase activity, necessary for HF-dependent remodeling, was reduced in ZDF rats compared with LZ rats and restored by ALT-711. Thus, targeting AGE cross-links may provide a therapeutic potential for overcoming microvascular complications in ischemic disorders occurring in diabetes.
Figures



References
-
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 - PubMed
-
- Goldstein BJ. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol 2002;90(5A):3G–10G - PubMed
-
- Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005;112:3066–3072 - PubMed
-
- Pieper GM, Siebeneich W. Diabetes-induced endothelial dysfunction is prevented by long-term treatment with the modified iron chelator, hydroxyethyl starch conjugated-deferoxamine. J Cardiovasc Pharmacol 1997;30:734–738 - PubMed
-
- Winer N, Sowers JR. Diabetes and arterial stiffening. Adv Cardiol 2007;44:245–251 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

