Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy
- PMID: 22416079
- PMCID: PMC3320264
- DOI: 10.1210/en.2011-2123
Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy
Abstract
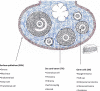
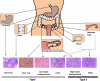
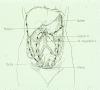
More than 90% of ovarian cancers have been thought to arise from epithelial cells that cover the ovarian surface or, more frequently, line subserosal cysts. Recent studies suggest that histologically similar cancers can arise from the fimbriae of Fallopian tubes and from deposits of endometriosis. Different histotypes are observed that resemble epithelial cells from the normal Fallopian tube (serous), endometrium (endometrioid), cervical glands (mucinous), and vaginal rests (clear cell) and that share expression of relevant HOX genes which drive normal gynecological differentiation. Two groups of epithelial ovarian cancers have been distinguished: type I low-grade cancers that present in early stage, grow slowly, and resist conventional chemotherapy but may respond to hormonal manipulation; and type II high-grade cancers that are generally diagnosed in advanced stage and grow aggressively but respond to chemotherapy. Type I cancers have wild-type p53 and BRCA1/2, but have frequent mutations of Ras and Raf as well as expression of IGFR and activation of the phosphatidylinositol-3-kinase (PI3K) pathway. Virtually all type II cancers have mutations of p53, and almost half have mutation or dysfunction of BRCA1/2, but other mutations are rare, and oncogenesis appears to be driven by amplification of several growth-regulatory genes that activate the Ras/MAPK and PI3K pathways. Cytoreductive surgery and combination chemotherapy with platinum compounds and taxanes have improved 5-yr survival, but less than 40% of all stages can be cured. Novel therapies are being developed that target high-grade serous cancer cells with PI3Kness or BRCAness as well as the tumor vasculature. Both in silico and animal models are needed that more closely resemble type I and type II cancers to facilitate the identification of novel targets and to predict response to combinations of new agents.
Figures
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. 2011. Global cancer statistics. CA Cancer J Clin 61:69–90 - PubMed
-
- Smith HO, Berwick M, Verschraegen CF, Wiggins C, Lansing L, Muller CY, Qualls CR. 2006. Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol 107:1075–1085 - PubMed
-
- Cheng W, Liu J, Yoshida H, Rosen D, Naora H. 2005. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med 11:531–537 - PubMed
-
- Soslow RA. 2008. Histologic subtypes of ovarian carcinoma: an overview. Int J Gynecol Pathol 27:161–174 - PubMed
-
- Silverberg SG. 2000. Histopathologic grading of ovarian carcinoma: a review and proposal. Int J Gynecol Pathol 19:7–15 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

