The retinoblastoma family of proteins and their regulatory functions in the mammalian cell division cycle
- PMID: 22417103
- PMCID: PMC3325851
- DOI: 10.1186/1747-1028-7-10
The retinoblastoma family of proteins and their regulatory functions in the mammalian cell division cycle
Abstract
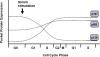
The retinoblastoma (RB) family of proteins are found in organisms as distantly related as humans, plants, and insects. These proteins play a key role in regulating advancement of the cell division cycle from the G1 to S-phases. This is achieved through negative regulation of two important positive regulators of cell cycle entry, E2F transcription factors and cyclin dependent kinases. In growth arrested cells transcriptional activity by E2Fs is repressed by RB proteins. Stimulation of cell cycle entry by growth factor signaling leads to activation of cyclin dependent kinases. They in turn phosphorylate and inactivate the RB family proteins, leading to E2F activation and additional cyclin dependent kinase activity. This propels the cell cycle irreversibly forward leading to DNA synthesis. This review will focus on the basic biochemistry and cell biology governing the regulation and activity of mammalian RB family proteins in cell cycle control.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

