Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus
- PMID: 22417684
- PMCID: PMC3327999
- DOI: 10.1042/BJ20120150
Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus
Abstract
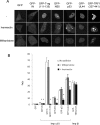
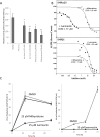
The movement of proteins between the cytoplasm and nucleus mediated by the importin superfamily of proteins is essential to many cellular processes, including differentiation and development, and is critical to disease states such as viral disease and oncogenesis. We recently developed a high-throughput screen to identify specific and general inhibitors of protein nuclear import, from which ivermectin was identified as a potential inhibitor of importin α/β-mediated transport. In the present study, we characterized in detail the nuclear transport inhibitory properties of ivermectin, demonstrating that it is a broad-spectrum inhibitor of importin α/β nuclear import, with no effect on a range of other nuclear import pathways, including that mediated by importin β1 alone. Importantly, we establish for the first time that ivermectin has potent antiviral activity towards both HIV-1 and dengue virus, both of which are strongly reliant on importin α/β nuclear import, with respect to the HIV-1 integrase and NS5 (non-structural protein 5) polymerase proteins respectively. Ivermectin would appear to be an invaluable tool for the study of protein nuclear import, as well as the basis for future development of antiviral agents.
Figures

References
-
- Hogarth C. A., Calanni S., Jans D. A., Loveland K. L. Importin α mRNAs have distinct expression profiles during spermatogenesis. Dev. Dyn. 2006;235:253–262. - PubMed
-
- Moseley G. W., Filmer R. P., DeJesus M. A., Jans D. A. Nucleocytoplasmic distribution of rabies virus P-protein is regulated by phosphorylation adjacent to C-terminal nuclear import and export signals. Biochemistry (Moscow) 2007;46:12053–12061. - PubMed
-
- Pryor M. J., Rawlinson S. M., Butcher R. E., Barton C. L., Waterhouse T. A., Vasudevan S. G., Bardin P. G., Wright P. J., Jans D. A., Davidson A. D. Nuclear localization of dengue virus nonstructural protein 5 through its importin α/β-recognized nuclear localization sequences is integral to viral infection. Traffic. 2007;8:795–807. - PubMed
-
- Forwood J. K., Jans D. A. Nuclear import pathway of the telomere elongation suppressor TRF1: inhibition by importin α. Biochemistry (Moscow) 2002;41:9333–9340. - PubMed
-
- Forwood J. K., Lam M. H., Jans D. A. Nuclear import of Creb and AP-1 transcription factors requires importin-β1 and Ran but is independent of importin-α. Biochemistry (Moscow) 2001;40:5208–5217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

