Spatio-temporal development of the endothelial glycocalyx layer and its mechanical property in vitro
- PMID: 22417911
- PMCID: PMC3405740
- DOI: 10.1098/rsif.2011.0901
Spatio-temporal development of the endothelial glycocalyx layer and its mechanical property in vitro
Abstract
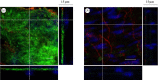
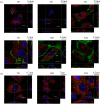
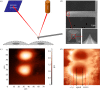
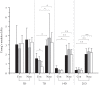
The endothelial glycocalyx is a thin layer of polysaccharide matrix on the luminal surface of endothelial cells (ECs), which contains sulphated proteoglycans and glycoproteins. It is a mechanotransducer and functions as an amplifier of the shear stress on ECs. It controls the vessel permeability and mediates the blood-endothelium interaction. This study investigates the spatial distribution and temporal development of the glycocalyx on cultured ECs, and evaluates mechanical properties of the glycocalyx using atomic force microscopy (AFM) nano-indentation. The glycocalyx on human umbilical vein endothelial cells (HUVECs) is observed under a confocal microscope. Manipulation of the glycocalyx is achieved using heparanase or neuraminidase. The Young's modulus of the cell membrane is calculated from the force-distance curve during AFM indentation. Results show that the glycocalyx appears predominantly on the edge of cells in the early days in culture, e.g. up to day 5 after seeding. On day 7, the glycocalyx is also seen in the apical area of the cell membrane. The thickness of the glycocalyx is approximately 300 nm-1 μm. AFM indentation reveals the Young's modulus of the cell membrane decreases from day 3 (2.93 ± 1.16 kPa) to day 14 (0.35 ± 0.15 kPa) and remains unchanged to day 21 (0.33 ± 0.19 kPa). Significant difference in the Young's modulus is also seen between the apical (1.54 ± 0.58 kPa) and the edge (0.69 ± 0.55 kPa) of cells at day 7. By contrast, neuraminidase-treated cells (i.e. without the glycocalyx) have similar values between day 3 (3.18 ± 0.88 kPa), day 14 (2.12 ± 0.78 kPa) and day 21 (2.15 ± 0.48 kPa). The endothelial glycocalyx in vitro shows temporal development in the early days in culture. It covers predominantly the edge of cells initially and appears on the apical membrane of cells as time progresses. The Young's modulus of the glycocalyx is deduced from Young's moduli of cell membranes with and without the glycocalyx layer. Our results show the glycocalyx on cultured HUVECs has a Young's modulus of approximately 0.39 kPa.
Figures

References
-
- Middleton J., Neil S., Wintle J., Clark-Lewis I., Moore H., Lam C., Auer M., Hub E., Rot A. 1997. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell 91, 385–39510.1016/S0092-8674(00)80422-5 (doi:10.1016/S0092-8674(00)80422-5) - DOI - DOI - PubMed
-
- Cai H., Harrison D. G. 2000. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 87, 840–84410.1161/01.RES.87.10.840 (doi:10.1161/01.RES.87.10.840) - DOI - DOI - PubMed
-
- Davignon J., Ganz P. 2004. Atherosclerosis: evolving vascular biology and clinical implications. Circulation 109, III-27–III-3210.1161/01.CIR.0000131515.03336.f8 (doi:10.1161/01.CIR.0000131515.03336.f8) - DOI - DOI - PubMed
-
- Squire J. M., Chew M., Nneji G., Neal C., Barry J., Michel C. 2001. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J. Struct. Biol. 136, 239–25510.1006/jsbi.2002.4441 (doi:10.1006/jsbi.2002.4441) - DOI - DOI - PubMed
-
- Luft J. H. 1966. Fine structure of capillary and endocapillary layer as revealed by ruthenium red. Federation Proc. 25, 1773–1783 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
