Gabapentin inhibits catecholamine release from adrenal chromaffin cells
- PMID: 22417967
- PMCID: PMC3341086
- DOI: 10.1097/ALN.0b013e31825153ea
Gabapentin inhibits catecholamine release from adrenal chromaffin cells
Abstract
Background: Gabapentin is most commonly prescribed for chronic pain, but acute perioperative effects, including preemptive analgesia and hemodynamic stabilization, have been reported. Adrenal chromaffin cells are a widely used model to investigate neurosecretion, and adrenal catecholamines play important physiologic roles and contribute to the acute stress response. However, the effects of gabapentin on adrenal catecholamine release have never been tested.
Methods: Primary cultures of bovine adrenal chromaffin cells were treated with gabapentin or vehicle for 18-24 h. The authors quantified catecholamine secretion from dishes of cells using high-performance liquid chromatography and resolved exocytosis of individual secretory vesicles from single cells using carbon fiber amperometry. Voltage-gated calcium channel currents were recorded using patch clamp electrophysiology and intracellular [Ca2+] using fluorescent imaging.
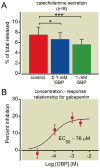
Results: Gabapentin produced statistically significant reductions in catecholamine secretion evoked by cholinergic agonists (24 ± 3%, n = 12) or KCl (16 ± 4%, n = 8) (mean ± SEM) but did not inhibit Ca2+ entry or calcium channel currents. Amperometry (n = 51 cells) revealed that gabapentin inhibited the number of vesicles released upon stimulation, with no change in quantal size or kinetics of these unitary events.
Conclusions: The authors show Ca2+ entry was not inhibited by gabapentin but was less effective at triggering vesicle fusion. The work also demonstrates that chromaffin cells are a useful model for additional investigation of the cellular mechanism(s) by which gabapentin controls neurosecretion. In addition, it identifies altered adrenal catecholamine release as a potential contributor to some of the beneficial perioperative effects of gabapentin.
Figures




Similar articles
-
Extracellular Ca²⁺ per se inhibits quantal size of catecholamine release in adrenal slice chromaffin cells.Cell Calcium. 2014 Sep;56(3):202-7. doi: 10.1016/j.ceca.2014.07.006. Epub 2014 Jul 22. Cell Calcium. 2014. PMID: 25103334
-
Inhibitory effects of opioids on voltage-dependent Ca(2+) channels and catecholamine secretion in cultured porcine adrenal chromaffin cells.Brain Res. 2002 Jun 28;942(1-2):11-22. doi: 10.1016/s0006-8993(02)02648-3. Brain Res. 2002. PMID: 12031848
-
Voltage inactivation of Ca2+ entry and secretion associated with N- and P/Q-type but not L-type Ca2+ channels of bovine chromaffin cells.J Physiol. 1999 Apr 15;516 ( Pt 2)(Pt 2):421-32. doi: 10.1111/j.1469-7793.1999.0421v.x. J Physiol. 1999. PMID: 10087342 Free PMC article.
-
Inhibition of Ca2+ channels and adrenal catecholamine release by G protein coupled receptors.Cell Mol Neurobiol. 2010 Nov;30(8):1201-8. doi: 10.1007/s10571-010-9596-7. Cell Mol Neurobiol. 2010. PMID: 21061161 Free PMC article. Review.
-
Calcium signaling and exocytosis in adrenal chromaffin cells.Physiol Rev. 2006 Oct;86(4):1093-131. doi: 10.1152/physrev.00039.2005. Physiol Rev. 2006. PMID: 17015485 Review.
Cited by
-
Rat Sympathetic Neuron Calcium Channels Are Insensitive to Gabapentin.Pharmaceuticals (Basel). 2024 Sep 19;17(9):1237. doi: 10.3390/ph17091237. Pharmaceuticals (Basel). 2024. PMID: 39338399 Free PMC article.
-
Sigma-1 receptor ligands inhibit catecholamine secretion from adrenal chromaffin cells due to block of nicotinic acetylcholine receptors.J Neurochem. 2017 Oct;143(2):171-182. doi: 10.1111/jnc.14149. Epub 2017 Sep 19. J Neurochem. 2017. PMID: 28815595 Free PMC article.
-
Gabapentin Modulates HCN4 Channel Voltage-Dependence.Front Pharmacol. 2017 Aug 21;8:554. doi: 10.3389/fphar.2017.00554. eCollection 2017. Front Pharmacol. 2017. PMID: 28871229 Free PMC article.
-
Butanol isomers exert distinct effects on voltage-gated calcium channel currents and thus catecholamine secretion in adrenal chromaffin cells.PLoS One. 2014 Oct 2;9(10):e109203. doi: 10.1371/journal.pone.0109203. eCollection 2014. PLoS One. 2014. PMID: 25275439 Free PMC article.
-
A microfluidic platform for chemical stimulation and real time analysis of catecholamine secretion from neuroendocrine cells.Lab Chip. 2013 Dec 7;13(23):4663-73. doi: 10.1039/c3lc50779c. Lab Chip. 2013. PMID: 24126415 Free PMC article.
References
-
- Kong VK, Irwin MG. Gabapentin: A multimodal perioperative drug? Br J Anaesth. 2007;99:775–86. - PubMed
-
- Fassoulaki A, Melemeni A, Paraskeva A, Petropoulos G. Gabapentin attenuates the pressor response to direct laryngoscopy and tracheal intubation. Br J Anaesth. 2006;96:769–73. - PubMed
-
- Memis D, Turan A, Karamanlioglu B, Seker S, Ture M. Gabapentin reduces cardiovascular responses to laryngoscopy and tracheal intubation. Eur J Anaesthesiol. 2006;23:686–90. - PubMed
-
- Derbyshire DR, Chmielewski A, Fell D, Vater M, Achola K, Smith G. Plasma catecholamine responses to tracheal intubation. Br J Anaesth. 1983;55:855–60. - PubMed
-
- Shribman AJ, Smith G, Achola KJ. Cardiovascular and catecholamine responses to laryngoscopy with and without tracheal intubation. Br J Anaesth. 1987;59:295–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

