Six-transmembrane topology for Golgi anti-apoptotic protein (GAAP) and Bax inhibitor 1 (BI-1) provides model for the transmembrane Bax inhibitor-containing motif (TMBIM) family
- PMID: 22418439
- PMCID: PMC3346125
- DOI: 10.1074/jbc.M111.336149
Six-transmembrane topology for Golgi anti-apoptotic protein (GAAP) and Bax inhibitor 1 (BI-1) provides model for the transmembrane Bax inhibitor-containing motif (TMBIM) family
Abstract
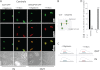
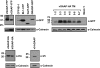
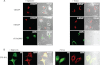
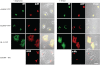
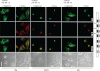
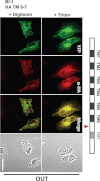
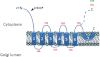
The Golgi anti-apoptotic protein (GAAP) is a hydrophobic Golgi protein that regulates intracellular calcium fluxes and apoptosis. GAAP is highly conserved throughout eukaryotes and some strains of vaccinia virus (VACV) and camelpox virus. Based on sequence, phylogeny, and hydrophobicity, GAAPs were classified within the transmembrane Bax inhibitor-containing motif (TMBIM) family. TMBIM members are anti-apoptotic and were predicted to have seven-transmembrane domains (TMDs). However, topology prediction programs are inconsistent and predicted that GAAP and other TMBIM members have six or seven TMDs. To address this discrepancy, we mapped the transmembrane topology of viral (vGAAP) and human (hGAAP), as well as Bax inhibitor (BI-1). Data presented show a six-, not seven-, transmembrane topology for vGAAP with a putative reentrant loop at the C terminus and both termini located in the cytosol. We find that this topology is also conserved in hGAAP and BI-1. This places the charged C terminus in the cytosol, and mutation of these charged residues in hGAAP ablated its anti-apoptotic function. Given the highly conserved hydrophobicity profile within the TMBIM family and recent phylogenetic data indicating that a GAAP-like protein may have been the ancestral progenitor of a subset of the TMBIM family, we propose that this vGAAP topology may be used as a model for the remainder of the TMBIM family of proteins. The topology described provides valuable information on the structure and function of an important but poorly understood family of proteins.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

