Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation
- PMID: 22419116
- PMCID: PMC3377774
- DOI: 10.1038/mi.2012.17
Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation
Abstract
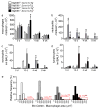
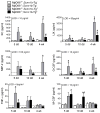
It has been postulated that mucus stasis is central to the pathogenesis of obstructive lung diseases. In Scnn1b-transgenic (Scnn1b-Tg⁺ mice, airway-targeted overexpression of the epithelial Na⁺ channel β subunit causes airway surface dehydration, which results in mucus stasis and inflammation. Bronchoalveolar lavage from neonatal Scnn1b-Tg⁺ mice, but not wild-type littermates, contained increased mucus, bacteria, and neutrophils, which declined with age. Scnn1b-Tg⁺ mice lung bacterial flora included environmental and oropharyngeal species, suggesting inhalation and/or aspiration as routes of entry. Genetic deletion of the Toll-interleukin-1 receptor adapter molecule MyD88 in Scnn1b-Tg⁺ mice did not modify airway mucus obstruction, but caused defective neutrophil recruitment and increased bacterial infection, which persisted into adulthood. Scnn1b-Tg⁺ mice derived into germ-free conditions exhibited mucus obstruction similar to conventional Scnn1b-Tg⁺ mice and sterile inflammation. Collectively, these data suggest that dehydration-induced mucus stasis promotes infection, compounds defects in other immune mechanisms, and alone is sufficient to trigger airway inflammation.
Conflict of interest statement
Figures





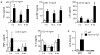
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

