A screening study of drug-drug interactions in cerivastatin users: an adverse effect of clopidogrel
- PMID: 22419147
- PMCID: PMC3830936
- DOI: 10.1038/clpt.2011.295
A screening study of drug-drug interactions in cerivastatin users: an adverse effect of clopidogrel
Abstract
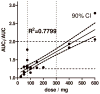
An analysis of a case-control study of rhabdomyolysis was conducted to screen for previously unrecognized cytochrome P450 enzyme (CYP) 2C8 inhibitors that may cause other clinically important drug-drug interactions. Medication use in cases of rhabdomyolysis using cerivastatin (n = 72) was compared with that in controls using atorvastatin (n = 287) for the period 1998-2001. The use of clopidogrel was strongly associated with rhabdomyolysis (odds ratio (OR) 29.6; 95% confidence interval (CI), 6.1-143). In a replication effort that used the US Food and Drug Administration (FDA) Adverse Event Reporting System (AERS), it was found that clopidogrel was used more commonly in patients with rhabdomyolysis receiving cerivastatin (17%) than in those receiving atorvastatin (0%, OR infinity; 95% CI = 5.2-infinity). Several medications were tested in vitro for their potential to cause drug-drug interactions. Clopidogrel, rosiglitazone, and montelukast were the most potent inhibitors of cerivastatin metabolism. Clopidogrel and its metabolites also inhibited cerivastatin metabolism in human hepatocytes. These epidemiological and in vitro findings suggest that clopidogrel may cause clinically important, dose-dependent drug-drug interactions with other medications metabolized by CYP2C8.
Figures

Comment in
-
The need for translational research on drug-drug interactions.Clin Pharmacol Ther. 2012 May;91(5):771-3. doi: 10.1038/clpt.2012.39. Clin Pharmacol Ther. 2012. PMID: 22513312 Free PMC article.
References
-
- Friedman MA, Woodcock J, Lumpkin MM, Shuren JE, Hass AE, Thompson LJ. The safety of newly approved medicines: do recent market removals mean there is a problem? JAMA. 1999;281:1728–34. - PubMed
-
- Food and Drug Administration. Guidance for Industry: Drug interaction studies – study design, data analysis, and implications for dosing and labeling. 2006 Sep;
-
- Psaty BM, Furberg CD, Ray WA, Weiss NS. Potential for conflict of interest in the evaluation of suspected adverse drug reactions: use of cerivastatin and risk of rhabdomyolysis. JAMA. 2004;292:2622–31. - PubMed
-
- Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL007902/HL/NHLBI NIH HHS/United States
- U01 HL080295/HL/NHLBI NIH HHS/United States
- HL085251/HL/NHLBI NIH HHS/United States
- R01 HL078888/HL/NHLBI NIH HHS/United States
- R01 HL087652/HL/NHLBI NIH HHS/United States
- N01-HC-85086/HC/NHLBI NIH HHS/United States
- HL078888/HL/NHLBI NIH HHS/United States
- R01HL087652/HL/NHLBI NIH HHS/United States
- N01-HC-85079/HC/NHLBI NIH HHS/United States
- N01 HC075150/HL/NHLBI NIH HHS/United States
- N01 HC045133/HC/NHLBI NIH HHS/United States
- N01 HC035129/HC/NHLBI NIH HHS/United States
- N01 HC015103/HC/NHLBI NIH HHS/United States
- N01 HC085086/HL/NHLBI NIH HHS/United States
- R01 HL085251/HL/NHLBI NIH HHS/United States
- N01 HC055222/HL/NHLBI NIH HHS/United States
- T32HL007902./HL/NHLBI NIH HHS/United States
- N01-HC45133/HC/NHLBI NIH HHS/United States
- N01-HC-55222/HC/NHLBI NIH HHS/United States
- N01 HC085079/HL/NHLBI NIH HHS/United States
- N01-HC-75150/HC/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

