Human recombinant activated factor VII for upper gastrointestinal bleeding in patients with liver diseases
- PMID: 22419301
- PMCID: PMC11569891
- DOI: 10.1002/14651858.CD004887.pub3
Human recombinant activated factor VII for upper gastrointestinal bleeding in patients with liver diseases
Abstract
Background: Mortality from upper gastrointestinal bleeding in patients with liver disease is high. Recombinant human activated factor VII (rHuFVIIa) has been suggested for patients with liver disease and upper gastrointestinal bleeding.
Objectives: To assess the beneficial and harmful effects of rHuFVIIa in patients with liver disease and upper gastrointestinal bleeding.
Search methods: We searched the Cochrane Hepato-Biliary Group Controlled Trials Register (December 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 4, 2011), MEDLINE (1948 to December 2011), EMBASE (1980 to December 2011), Science Citation Index Expanded (1900 to December 2011), and LILACS (December 2011). We sought additional randomised trials from the reference lists of the trials and reviews identified through the electronic searches.
Selection criteria: Randomised clinical trials.
Data collection and analysis: Outcome data from randomised clinical trials were extracted and were presented using random-effects model meta-analyses. Data on the risk of bias in the included trials were also extracted.
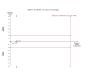
Main results: We included two trials with 493 randomised participants with various Child-Pugh scores. The trials had a low risk of bias. The rHuFVIIa administration did not reduce the risk of mortality within five days (21/288 (7.3%) versus 15/205 (7.3%); risk ratio (RR) 0.88, 95% confidence interval (CI) 0.48 to 1.64, I(2) = 49%) and within 42 days (5/286 (1.7%) versus 36/205 (17.6%); RR 1.01, 95% CI 0.55 to 1.87, I(2) = 55%) when compared with placebo. Trial sequential analysis demonstrated that there is sufficient evidence to exclude that rHuFVIIa decreases mortality by 80%, but there is insufficient evidence to exclude smaller effects. The rHuFVIIa did not increase the risk of adverse events by number of patients (218/297 (74%) and 164/210 (78%); RR 0.94, 95% CI 0.84 to 1.04, I(2) = 1%), serious adverse events by adverse events reported (164/590 (28%) versus 123/443 (28%); RR 0.91, 95% CI 0.75 to 1.11, I(2) = 0%), and thromboembolic adverse events (16/297 (5.4%) versus 14/210 (6.7%); RR 0.80, 95% CI 0.40 to 1.60, I(2) = 0%) when compared with placebo.
Authors' conclusions: We found no evidence to support or reject the administration of rHuFVIIa for patients with liver disease and upper gastrointestinal bleeding. Further adequately powered randomised clinical trials are needed in order to evaluate the proper role of rHuFVIIa for treating upper gastrointestinal bleeding in patients with liver disease. Although the results are based on trials with low risk of bias, the heterogeneity and the small sample size result in rather large confidence intervals that cannot exclude the possibility that the intervention has some beneficial or harmful effect. Further trials with alow risk of bias are required to make more confident conclusions about the effects of the intervention.
Conflict of interest statement
In 2004, Arturo Martí‐Carvajal was employed by Eli Lilly to run a four‐hour workshop on 'How to critically appraise clinical trials on osteoporosis and how to teach this'. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
In 2007, Arturo Martí‐Carvajal was employed by Merck to run a four‐hour workshop 'How to critically appraise clinical trials and how to teach this'. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
Georgia Salanti and Despoina‐Elvira Karakitsiou do not have any conflict of interest.
Figures














Update of
-
Human recombinant activated factor VII for upper gastrointestinal bleeding in patients with liver diseases.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD004887. doi: 10.1002/14651858.CD004887.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2012 Mar 14;(3):CD004887. doi: 10.1002/14651858.CD004887.pub3. PMID: 17253529 Updated.
References
References to studies included in this review
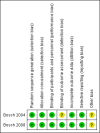
Bosch 2004 {published data only}
-
- Bosch J, Thabut D, Bendtsen F, D'Amico G, Albillos A, Gonzalez Abraldes J, et al. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double‐blind trial. Gastroenterology 2004;127:1123‐30. - PubMed
-
- Thabut D, Franchis R, Bendtsen F, D'Amico G, Albillos A, Abraldes JG, et al. Efficacy of activated recombinant factor VII (RFVIIA; Novoseven®) in cirrhotic patients with upper gastrointestinal bleeding: a randomised placebo‐controlled double‐blind multicenter trial. Journal of Hepatology 2003;38 Suppl 2:13.
Bosch 2008 {published data only}
-
- Bosch J, Thabut D, Albillos A, Carbonell N, Spicak J, Massard J, International Study Group on rFVIIa in UGI Hemorrhage. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: A randomized, controlled trial. Hepatology 2008;47:1604‐14. - PubMed
References to studies excluded from this review
Arellano‐Rodrigo 2011 {published data only}
-
- Arellano‐Rodrigo E. Safety of recombinant activated factor VII in randomized clinical trials. The New England Journal of Medicine 2011; Vol. 364, issue 6:574‐5; author reply 575‐6. - PubMed
Bosch 2005 {published data only}
-
- Bosch J, Thabut D, Bendtsen F, Russo MW. Factor VIIA for upper GI bleeding in cirrhotic patients: It is all in the study design. Evidence‐Based Gastroenterology 205;6:22‐3.
Ejlersen 2001 {published data only}
-
- Ejlersen E, Melsen T, Ingerslev J, Andreasen RB, Vilstrup H. Recombinant activated factor VII (rFVIIa) acutely normalizes prothrombin time in patients with cirrhosis during bleeding from oesophageal varices. Scandinavian Journal of Gastroenterology 2001;36:1081‐5. - PubMed
Flower 2010 {published data only}
-
- Flower O, Phillips LE, Cameron P, Gunn K, Dunkley S, Watts A, et al. Recombinant activated factor VII in liver patients: a retrospective cohort study from Australia and New Zealand. Blood Coagulation & Fibrinolysis 2010;21:207‐15. - PubMed
Franchini 2008 {published data only}
-
- Franchini M, Montagnana M, Targher G, Zaffanello M, Lippi G. The use of recombinant factor VIIa in liver diseases. Blood Coagulation & Fibrinolysis 2008;19:341‐8. - PubMed
Levi 2010 {published data only}
-
- Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. The New England Journal of Medicine 2010;363(19):1791‐800. - PubMed
Martí‐Carvajal 2007 {published data only}
Plessier 2004 {published data only}
-
- Plessier A. Evaluation and non‐specific management of upper gastrointestinal bleeding due to rupture of esophageal varices [Évaluation et prise en charge non spécifique de l'hémorragie digestive par rupture de varices oesophagiennes]. Gastroentérologie Clinique et Biologique 2004;28:B9‐B14. - PubMed
Romero‐Castro 2004 {published data only}
-
- Romero‐Castro R, Jimenez‐Saenz M, Pellicer‐Bautista F, Gomez‐Parra M, Arguelles Arias F, Guerrero‐Aznar MD, et al. Recombinant‐activated factor VII as hemostatic therapy in eight cases of severe hemorrhage from esophageal varices. Clinical Gastroenterology and Hepatology 2004;2:78‐84. - PubMed
Thabut 2011 {published data only}
-
- Thabut D, Rudler M, Rousseau G, Poynard T. Recombinant activated factor VII in chronic liver diseases: should we be afraid of thromboembolic events?. Journal of Hepatology 2011;55(2):483‐5. - PubMed
Vilstrup 2006 {published data only}
-
- Vilstrup H, Markiewicz M, Biesma D, Brozovic VV, Laminoga N, Malik M, et al. Recombinant activated factor VII in an unselected series of cases with upper gastrointestinal bleeding. Thrombosis Research 2006;118:595‐601. - PubMed
Additional references
Afessa 2000
-
- Afessa B, Kubilis PS. Upper gastrointestinal bleeding in patients with hepatic cirrhosis: clinical course and mortality prediction. American Journal of Gastroenterology 2000;95:484‐9. - PubMed
Aitken 2004
-
- Aitken MG. Recombinant factor VIIa. Emergency Medicine Australasia 2004;16(5‐6):446‐55. [PUBMED: 15537408] - PubMed
Allen 2002
-
- Allen GA, Hoffman M, Roberts HR, Monroe DM 3rd. Recombinant activated factor VII: its mechanism of action and role in the control of hemorrhage. Canadian Journal of Anaesthesia 2002;49 Suppl:7‐14. - PubMed
Altman 1995
Amitrano 2002
-
- Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Seminars in Liver Disease 2002;22:83‐96. - PubMed
Bernstein 1997
-
- Bernstein DE, Jeffers L, Erhardtsen E, Reddy KR, Glazer S, Squiban P, et al. Recombinant factor VIIa corrects prothrombin time in cirrhotic patients: a preliminary study. Gastroenterology 1997;113(6):1930‐7. - PubMed
Boutron 2010
-
- Boutron I, Dutton S, Ravaud P, Altman DG. Reporting and interpretation of randomized controlled trials with statistically nonsignificant results for primary outcomes. JAMA 2010;26:2058‐64. - PubMed
Brown 2003
-
- Brown JB, Emerick KM, Brown DL, Whitington PF, Alonso EM. Recombinant factor VIIa improves coagulopathy caused by liver failure. Journal of Pediatric Gastroenterology and Nutrition 2003;37:268‐72. - PubMed
Czernichow 2000
-
- Czernichow P, Hochain P, Nousbaum JB, Raymond JM, Rudelli A, Dupas JL, et al. Epidemiology and course of acute upper gastro‐intestinal haemorrhage in four French geographical areas. European Journal of Gastroenterology & Hepatology 2000;12:175‐81. - PubMed
Dallal 2001
del Olmo 2000
-
- Olmo JA, Pena A, Serra MA, Wassel AH, Benages A, Rodrigo JM. Predictors of morbidity and mortality after the first episode of upper gastrointestinal bleeding in liver cirrhosis. Journal of Hepatology 2000;32:19‐24. - PubMed
Erhardtsen 2000
-
- Erhardtsen E. Pharmacokinetics of recombinant activated factor VII (rFVIIa). Seminars in Thrombosis and Hemostasis 2000;26(4):385‐91. - PubMed
Erhardtsen 2002
-
- Erhardtsen E. Ongoing NovoSeven trials. Intensive Care Medicine 2002;28 Suppl:248‐55. - PubMed
Fermi Paradox
-
- Fermi Paradox. www.crystalinks.com/fermiparadox.html (accessed 20 December 2011).
Franchini 2010
-
- Franchini M, Lippi G. Recombinant activated factor VII: mechanisms of action and current indications. Seminars in Thrombosis and Hemostasis 2010;36:485‐92. - PubMed
Freiman 1978
-
- Freiman JA, Chalmers TC, Smith H Jr, Kuebler RR. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 "negative" trials. New England Journal of Medicine 1978;299:690‐4. - PubMed
Gluud 2011
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2011, Issue 12. Art. No.: LIVER.
Green 2002
-
- Green SB. Design of randomized trials. Epidemiologic Reviews 2002;24:4‐11. - PubMed
Green 2003
-
- Green BT, Rockey DC. Acute gastrointestinal bleeding. Seminars in Gastrointestinal Disease 2003;14(2):44‐65. - PubMed
Guyatt 2008
Hebert 2002
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Ioannidis 2010
-
- Ioannidis JP. Meta‐research: The art of getting it wrong. Research Synthesis Methods 2010;10(3‐4):169‐84. - PubMed
Kirby 2002
-
- Kirby A, Gebski V, Keech AC. Determining the sample size in a clinical trial. The Medical Journal of Australia 2002;177:256‐7. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses. Lancet 1998;352:609‐13. - PubMed
NERI 2003
-
- New England Research Institutes (NERI). The positives of negative results. http://www.neriscience.com/Portals/0/Uploads/Documents/fall2003.pdf (accessed 20 December 2011).
O'Connell 2006
-
- O'Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA 2006;295:293‐8. - PubMed
O'Hara 2006
-
- O'Hara B, Fowler MS, Johnson CA. Why negatives should be viewed as positives. Nature 2006;439:782. - PubMed
Paramo 1993
-
- Paramo JA, Rocha E. Hemostasis in advanced liver disease. Seminars in Thrombosis and Hemostasis 1993;19:184‐90. - PubMed
Pavese 2005
-
- Pavese P, Bonadona A, Beaubien J, Labrecque P, Pernod G, Letoublon C, et al. FVIIa corrects the coagulopathy of fulminant hepatic failure but may be associated with thrombosis: a report of four cases. Canadian Journal of Anaesthesia 2005;52:26‐9. - PubMed
Protell 1981
-
- Protell RL, Silverstein FE, Gilbert DA, Feld AD. Severe upper gastrointestinal bleeding. Part I: Causes, pathogenesis and methods of diagnosis. Clinics in Gastroenterology 1981;10:17‐26. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2011.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Silva de Lima 2004
-
- Silva de Lima M, Garcia de Oliveira Soares B. The value of publishing negative results from a randomized controlled trial: the Rosenheck's study [O valor de publicar‐se resultados negativos de ensaios clínicos randomizados: o estudo de Rosenheck]. Revista Brasileira de Psiquiatria 2004;26:135. - PubMed
Tonda 2003
-
- Tonda R, Galan AM, Pino M, Cirera I, Bosch J, Hernandez MR, et al. Hemostatic effect of activated recombinant factor VII (rFVIIa) in liver disease: studies in an in vitro model. Journal of Hepatology 2003;39:954‐9. - PubMed
Tripodi 2005
-
- Tripodi A, Salerno F, Chantarangkul V, Clerici M, Cazzaniga M, Primignani M, et al. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology 2005;41:553‐8. - PubMed
TSA 2011 [Computer program]
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. Trial Sequential Analysis. Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research, 2011.
Van Thiel 2005
-
- Thiel DH, Farr DE, Mindikoglu AL, Todo A, George MM. Recombinant human factor VIIa‐induced alterations in tissue factor and thrombomodulin in patients with advanced liver cirrhosis. Journal of Gastroenterology and Hepatology 2005;20:882‐9. - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

