Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia
- PMID: 22419303
- PMCID: PMC11930396
- DOI: 10.1002/14651858.CD005011.pub4
Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia
Abstract
Background: Recombinant factor VIIa (rFVIIa) is licensed for use in patients with haemophilia and inhibitory allo-antibodies and for prophylaxis and treatment of patients with congenital factor VII deficiency. It is also used for off-license indications to prevent bleeding in operations where blood loss is likely to be high, and/or to stop bleeding that is proving difficult to control by other means. This is the third version of the 2007 Cochrane review on the use of recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia, and has been updated to incorporate recent trial data.
Objectives: To assess the effectiveness of rFVIIa when used therapeutically to control active bleeding or prophylactically to prevent (excessive) bleeding in patients without haemophilia.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and other medical databases up to 23 March 2011.
Selection criteria: Randomised controlled trials (RCTs) comparing rFVIIa with placebo, or one dose of rFVIIa with another, in any patient population (except haemophilia). Outcomes were mortality, blood loss or control of bleeding, red cell transfusion requirements, number of patients transfused and thromboembolic adverse events.
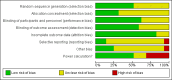
Data collection and analysis: Two authors independently assessed potentially relevant studies for inclusion, extracted data and examined risk of bias. We considered prophylactic and therapeutic rFVIIa studies separately.
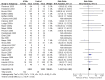
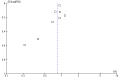
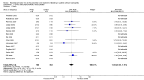
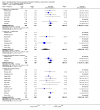
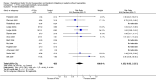
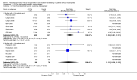
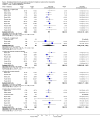
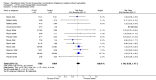
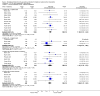
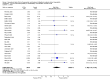
Main results: Twenty-nine RCTs were included: 28 were placebo-controlled, double-blind RCTs and one compared different doses of rFVIIa. In the 'Risk of bias' assessment, most studies were found to have some threats to validity although therapeutic RCTs were found to be less prone to bias than prophylactic RCTs.Sixteen trials involving 1361 participants examined the prophylactic use of rFVIIa; 729 received rFVIIa. There was no evidence of mortality benefit (risk ratio (RR) 1.04; 95% confidence interval (CI) 0.55 to 1.97). There was decreased blood loss (mean difference (MD) -297 mL; 95% CI -416 to -178) and decreased red cell transfusion requirements (MD -261 mL; 95% CI -367 to -154) with rFVIIa treatment; however, these values were likely overestimated due to the inability to incorporate data from trials (four RCTs in the outcome of blood loss and three RCTs in the outcome of transfusion requirements) showing no difference of rFVIIa treatment compared to placebo. There was a trend in favour of rFVIIa in the number of participants transfused (RR 0.85; 95% CI 0.72 to 1.01). However, there was a trend against rFVIIa with respect to thromboembolic adverse events (RR 1.35; 95% CI 0.82 to 2.25).Thirteen trials involving 2929 participants examined the therapeutic use of rFVIIa; 1878 received rFVIIa. There were no outcomes where any observed advantage or disadvantage of rFVIIa over placebo could not have been observed by chance alone. There was a trend in favour of rFVIIa for reducing mortality (RR 0.91; 95% CI 0.78 to 1.06). However, there was a trend against rFVIIa for increased thromboembolic adverse events (RR 1.14; 95% CI 0.89 to 1.47).When all trials were pooled together to examine the risk of thromboembolic events, a significant increase in total arterial events was observed (RR 1.45; 95% CI 1.02 to 2.05).
Authors' conclusions: The effectiveness of rFVIIa as a more general haemostatic drug, either prophylactically or therapeutically, remains unproven. The results indicate increased risk of arterial events in patients receiving rFVIIa. The use of rFVIIa outside its current licensed indications should be restricted to clinical trials.
Conflict of interest statement
Yulia Lin: The author is a study site investigator for a registry on the off‐label use of rFVIIa in Canada funded by an unrestricted educational grant from Novo Nordisk but receives no personal financial payments for participation in the registry.
Chris Hyde was an employee of NHS Blood and Transplant when this review was commenced. This arrangement ended in 2009.
The other authors have no declarations of interest.
Figures























Update of
-
Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia.Cochrane Database Syst Rev. 2011 Feb 16;(2):CD005011. doi: 10.1002/14651858.CD005011.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2012 Mar 14;(3):CD005011. doi: 10.1002/14651858.CD005011.pub4. PMID: 21328270 Updated.
References
References to studies included in this review
Boffard 2005a {published data only}
-
- Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, et al. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized placebo‐controlled double blind clinical trials. Journal of Trauma 2005;59(1):8‐15. - PubMed
Boffard 2005b {published data only}
-
- Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, et al. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized placebo‐controlled double blind clinical trials. Journal of Trauma 2005;59(1):8‐15. - PubMed
Bosch 2004 {published data only}
-
- Bosch J, Thabut D, Bendtsen F, D'Amico G, Albillos A, Gonzalez Abraldes J, et al. Recombinant factor VIIa for upper gastrointestinal bleeding patients with cirrhosis: a randomized, double‐blind trial. Gastroenterology 2004;127(4):1123‐30. - PubMed
Bosch 2008 {published data only}
-
- Bosch J, Thabut D, Albillos A, Carbonell N, Spicak J, Massard J, et al. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: a randomized, controlled trial. Hepatology 2008;47(5):1604‐14. - PubMed
Chuansumrit 2005 {published data only}
-
- Chuansumrit A, Wangruangsatid S, Lektrakul Y, Chua MN, Zeta Capeding MR, Bech OM, et al. Control of bleeding in children with Dengue hemorrhagic fever using recombinant activated factor VII: a randomized, double‐blind, placebo‐controlled study. Blood Coagulation & Fibrinolysis 2005;16(8):549‐55. - PubMed
Diprose 2005 {published data only}
-
- Diprose P, Herbertson M, O'Shaughnessy D, Gill R. A pilot double blind randomized placebo‐controlled trial of the use of recombinant factor VIIa (rf VIIa) in high transfusion risk cardiac surgery. European Journal of Anaesthesiology 2005;21(Suppl 33):2‐36.
-
- Herbertson M. Recombinant activated factor VII in cardiac surgery. Blood Coagulation & Fibrinolysis 2004;15(Suppl 1):S31‐2. - PubMed
Ekert 2006 {published data only}
-
- Ekert H, Brizard C, Eyers R, Cochrane A, Henning R. Elective administration in infants of low‐dose recombinant activated factor VII (rFVIIa) in cardiopulmonary bypass surgery for congenital heart disease does not shorten time to chest closure or reduce blood loss and need for transfusions: a randomized, double‐blind, parallel group, placebo‐controlled study of rFVIIa and standard haemostatic replacement therapy versus standard haemostatic replacement therapy. Blood Coagulation & Fibrinolysis 2006;17(5):389‐95. - PubMed
Essam 2007 {published data only}
-
- Essam MA. Prophylactic administration of recombinant activated factor VII in coronary revascularization surgery. Internet Journal of Anesthesiology 2007;13(1):10.
Friederich 2003 {published data only}
-
- Friederich PW, Geerdink MGF, Keller TT, Messelink EJ, Henny Ch P, Levi M. Novel applications of recombinant factor VIIa: the effect of the administration of recombinant activated factor VII on perioperative blood loss in patients undergoing transabdominal retropubic prostatectomy: the PROSE study. Infusionstherapie und Transfusionsmedizin 2001;28(2):112‐3.
-
- Friederich PW, Geerdink MGF, Spataro M, Messelink EJ, Henny Ch P, Buller HR, et al. The effect of the administration of recombinant activated factor VII on perioperative blood loss in patients undergoing transabdominal retropubic prostatectomy: the PROSE study. Blood Coagulation & Fibrinolysis 2000;11(Suppl 1):S129‐32. - PubMed
-
- Friederich PW, Keller T, Buller HR, Levi M. Effect of recombinant activated factor VII on perioperative blood loss in patients undergoing retropubic prostatectomy: a double‐blind placebo‐controlled randomised trial. Lancet 2003;361(9353):201‐5. - PubMed
Gill 2009 {published data only}
-
- Gill R, Herbertson M, Vuylsteke A, Olsen P S, Heymann C, Mythen M, et al. Additional data obtained from author 2009.
-
- Gill R, Herbertson M, Vuylsteke A, Olsen PS, Heymann C, Mythen M, et al. Safety and efficacy of recombinant activated factor VII: a randomized placebo‐controlled trial in the setting of bleeding after cardiac surgery. Circulation 2009;120(1):21‐7. - PubMed
Hanna 2010 {published data only}
-
- Hanna MG, Refaie A, Gouda N, Obaya G. Reduction of peri‐operative bleeding in craniofacial surgeries in pediatrics. Comparison between recombinant factor VII and tranexamic acid. Egyptian Journal of Anaesthesia 2010;26:53‐61.
Hauser 2010a {published data only}
-
- Hauser C J, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, et al. Results of the CONTROL trial: efficacy and safety of recombinant activated factor VII in the management of refractory traumatic hemorrhage. Journal of Trauma 2010;69(3):489‐500. - PubMed
Hauser 2010b {published data only}
-
- Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, et al. Results of the CONTROL trial: efficacy and safety of recombinant activated factor VII in the management of refractory traumatic hemorrhage. Journal of Trauma 2010;69(3):489‐500. - PubMed
Jeffers 2002 {published data only}
-
- Jeffers L, Chalasani N, Balart L, Pyrsopoulos N, Erhardtsen E. Safety and efficacy of recombinant factor VIIa in patients with liver disease undergoing laparoscopic liver biopsy. Gastroenterology 2002;123:118‐26. - PubMed
Johansson 2007 {published data only}
-
- Johansson PI, Eriksen K, Nielsen SL, Rojkjaer R, Alsbjorn B. Recombinant FVIIa decreases perioperative blood transfusion requirement in burn patients undergoing excision and skin grafting ‐ results of a single centre pilot study. Burns 2007;33(4):435‐40. - PubMed
Lodge 2005a {published data only}
-
- Lodge JP, Jonas S, Oussoultzoglou E, Malago M, Jayr C, Cherqui D, et al. Recombinant coagulation factor VIIa in major liver resection: a randomized, placebo‐controlled, double‐blind clinical trial. Anesthesiology 2005;102(2):269. - PubMed
-
- Lodge JPA. Hemostasis in liver resection surgery. Seminars in Hematology 2004;41(Suppl 1):70‐5. - PubMed
Lodge 2005b {published data only}
-
- Lodge JP, Jonas S, Jones RM, Olausson M, Mir‐Pallardo J, Soefelt S, et al. Efficacy and safety of repeated perioperative doses of recombinant factor VIIa in liver transplantation. Liver Transplantation 2005;11(8):973‐9. - PubMed
-
- Lodge JPA. Hemostasis in liver resection surgery. Seminars in Hematology 2004;41(Suppl 1):70‐5. - PubMed
Ma 2006 {published data only}
-
- Ma B, Wang ZN, Zhang BR, Xu ZY, Yang LX, Chen KB, et al. Effect of recombinant activated factor VIIa on early recovery of patients undergoing cardiac valve replacement under cardiopulmonary bypass: a randomized double‐blind placebo‐controlled trial. Academic Journal of Second Military Medical University 2006;27:1110‐3.
Mayer 2005a {published data only}
-
- Mayer SA. Intracerebral hemorrhage: natural history and rationale of ultra‐early hemostatic therapy. Intensive Care Medicine 2002;28:S235‐40. - PubMed
-
- Mayer SA, Nikolai MD, Brun C, Begtrup K, Broderick J, Davis S, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. New England Journal of Medicine 2005;352(8):777‐85. - PubMed
-
- Pickard JD, Kirkpatrick PJ, Melsen T, Andreasen RB, Gelling L, Fryer T, et al. Potential role of NovoSeven in the prevention of rebleeding following aneurysmal subarachnoid haemorrhage. Blood Coagulation & Fibrinolysis 2000;11(Suppl 1):S117‐20. - PubMed
Mayer 2005b {published data only}
-
- Mayer SA. Intracerebral hemorrhage: natural history and rationale of ultra‐early hemostatic therapy. Intensive Care Medicine 2002;28:S235‐40. - PubMed
-
- Mayer SA, Brun NC, Broderick J, Davis S, Diringer MN, Skolnick BE, et al. Safety and feasibility of recombinant factor VIIa for acute intracerebral hemorrhage. Stroke 2005;36(1):74‐9. - PubMed
-
- Pickard JD, Kirkpatrick PJ, Melsen T, Andreasen RB, Gelling L, Fryer T, et al. Potential role of NovoSeven in the prevention of rebleeding following aneurysmal subarachnoid haemorrhage. Blood Coagulation & Fibrinolysis 2000;11(Suppl 1):S117‐20. - PubMed
Mayer 2006 {published data only}
-
- Mayer SA, Brun NC, Broderick J, Davis SM, Diringer MN, Skolnick BE, et al. Recombinant activated factor VII for acute intracerebral hemorrhage: US phase IIA trial. Neurocritical Care 2006;4(3):206‐14. - PubMed
Mayer 2008 {published data only}
-
- Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. New England Journal of Medicine 2008;358(20):2127‐37. - PubMed
Narayan 2008 {published data only}
-
- Narayan RK, Maas AI, Marshall LF, Servadei F, Skolnick BE, Tillinger MN, rFVIIa Traumatic ICH Study Group. Recombinant factor VIIA in traumatic intracerebral hemorrhage: results of a dose‐escalation clinical trial. Neurosurgery 2008;62(4):776‐86. [Clinicaltrials.gov Identifier: NCT00123591] - PubMed
Pihusch 2005 {published data only}
-
- Bacigalupo A. Haemopoietic stem cell transplants: the impact of haemorrhagic complications. Blood Reviews 2003;17:S6‐10. - PubMed
-
- Pihusch M, Bacigalupo A, Szer J, Depka Prondzinski M, Gaspar‐Blaudschun B, Hyveled L, et al. Recombinant activated factor VII in treatment of bleeding complications following hematopoietic stem cell transplantation. Journal of Thrombosis & Haemostasis 2005;3(9):1935‐44. - PubMed
Planinsic 2005 {published data only}
-
- Planinsic RM, Testa G, Grande L, Candela A, Meer J, Porte RJ, et al. Safety and efficacy of a single bolus administration of recombinant factor VIIa in liver transplantation due to chronic liver disease. Liver Transplantation 2005;11(8):895‐900. - PubMed
Pugliese 2007 {published data only}
-
- Pugliese F, Ruberto F, Summonti D, Perrella S, Cappannoli A, Tosi A, et al. Activated recombinant factor VII in orthotopic liver transplantation. Transplantation Proceedings 2007;39(6):1883‐5. - PubMed
Raobaikady 2005 {published data only}
-
- Raobaikady R, Redman J, Ball JA, Maloney G, Grounds RM. Use of activated recombinant coagulation factor VII in patients undergoing reconstruction surgery for traumatic fracture of pelvis and acetabulum: a double‐blind randomized, placebo‐controlled trial. British Journal of Anaesthesia 2005;94(5):586‐91. - PubMed
Sachs 2007 {published data only}
-
- Sachs B, Delacy D, Green J, Graham RS, Ramsay J, Kreisler N, et al. Recombinant activated factor VII in spinal surgery: a multicenter, randomized, double‐blind, placebo‐controlled, dose‐escalation trial. Spine 2007;32(21):2285‐93. - PubMed
Shao 2006 {published data only}
-
- Shao YF, Yang JM, Chau GY, Sirivatanauksorn Y, Zhong SX, Erhardtsen E, et al. Safety and hemostatic effect of recombinant activated factor VIIa in cirrhotic patients undergoing partial hepatectomy: a multicenter, randomized, double‐blind, placebo‐controlled trial. American Journal of Surgery 2006;191(2):245‐9. - PubMed
References to studies excluded from this review
Ashrani 2006 {published data only}
-
- Ashrani AA, Gabriel DA, Gajewski JL, Jacobs DRJ, Weisdorf DJ, Key NS. Pilot study to test the efficacy and safety of activated recombinant factor VII (NovoSeven) in the treatment of refractory hemorrhagic cystitis following high‐dose chemotherapy. Bone Marrow Transplantation 2006;38(12):825‐8. - PubMed
Bijsterveld 2002 {published data only}
-
- Bijsterveld NR, Moons AH, Boekholdt SM, Aken BE, Fennema H, Peters RJ, et al. Ability of recombinant factor VIIa to reverses the anticoagulant effect of the pentasaccharide fondaparinux in healthy volunteers. Circulation 2002;106(20):2550‐4. - PubMed
Bijsterveld 2004 {published data only}
-
- Bijsterveld NR, Vink R, Arken BE, Fennema H, Peters RJG, Meijers JCM, et al. Recombinant factor VIIa reverses the anticoagulant effect of the long‐acting pentasaccharide idraparinux in healthy volunteers. British Journal of Haematology 2004;124(5):653‐8. - PubMed
Boffard 2009 {published data only}
-
- Boffard KD, Choong PI, Kluger Y, Riou B, Rizoli SB, Rossaint R, et al. NovoSeven Trauma Study Group. The treatment of bleeding is to stop the bleeding! Treatment of trauma‐related hemorrhage. Transfusion 2009;49(Suppl 5):240s‐7s. - PubMed
Bysted 2007 {published data only}
-
- Bysted BV, Scharling B, Møller T, Hansen BL. A randomized, double‐blind trial demonstrating bioequivalence of the current recombinant activated factor VII formulation and a new robust 25 degrees C stable formulation. Haemophilia 2007;13(5):527‐32. - PubMed
Davis 2004 {published data only}
-
- Davis S, Mayer S, Brun N, Broderick J, Diringer MN, Steiner T. Safety of activated recombinant factor VII in acute intracerebral hemorrhage: results of two multi‐center placebo‐controlled trials. Internal Medicine Journal 2004;34:1‐2.
Diringer 2007 {published data only}
-
- Diringer MN, Ferran JM, Broderick JP, Davis S, Mayer SA, Steiner T, et al. Impact of recombinant activated factor VII on health‐related quality of life after intracerebral hemorrhage. Cerebrovascular Diseases 2007;23(2‐3):219‐25. - PubMed
Elgafy 2010 {published data only}
-
- Elgafy H, Bransford RJ, McGuire RA, Dettori JR, Fischer D. Blood loss in major spine surgery: are there effective measures to decrease massive hemorrhage in major spine fusion surgery?. Spine 2010;35(Suppl 9):S47‐56. [PUBMED: 20407351] - PubMed
Ensor 2011 {published data only}
-
- Ensor CR, Paciullo CA, Cahoon WD Jr, Nolan PE Jr. Pharmacotherapy for mechanical circulatory support: a comprehensive review. Annals of Pharmacotherapy 2011;45(1):60‐77. [PUBMED: 21205950] - PubMed
Fridberg 2005 {published data only}
-
- Fridberg MJ, Hedner U, Roberts HR, Erhardtsen E. A study of the pharmacokinetics and safety of recombinant activated factor VII in healthy Caucasian and Japanese subjects. Blood Coagulation and Fibrinolysis 2005;16(4):259‐66. - PubMed
Gurusamy 2009 {published data only}
Jilma 2002 {published data only}
-
- Jilma B, Marsil C, Mayr F, Graninger MT, Taylor FB, Ribel MC, et al. Pharmacodynamics of active site‐inhibited factor VIIa in endotoxin‐induced coagulation in humans. Clinical Pharmacology and Therapeutics 2002;72(4):403‐10. - PubMed
Johansson 2010 {published data only}
Kolban 2005 {published data only}
-
- Kolban M, Balachowska KI, Chmielnicki M. The use of recombinant coagulation factor VIIa in patients undergoing surgical correction of scoliosis with the C‐D method. Ortopedia Traumatologia Rehabilitacja 2005;7(3):285‐9. - PubMed
Larsen 2010 {published data only}
-
- Larsen OH, Ezban M, Persson E, Ingerslev J, Sorensen B. Artificial contact pathway activation masks the haemostatic potential of rFVIIa and NN1731 in thrombocytopenic whole blood. British Journal of Haematology 2010;150(1):124‐7. - PubMed
Leduc 2009 {published data only}
-
- Leduc D, Senikas V, Lalonde AB, Ballerman C, Biringer A, Delaney M, et al. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. Journal of Obstetrics and Gynaecology 2009;31(10):980‐93. [PUBMED: 19941729] - PubMed
Levi 2010 {published data only}
-
- Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. New England Journal of Medicine 2010;363(19):1791‐800. [PUBMED: 21047223] - PubMed
Lin 2011b {published data only}
Logan 2010 {published data only}
-
- Logan AC, Goodnough LT. Recombinant factor VIIa: an assessment of evidence regarding its efficacy and safety in the off‐label setting. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. Education Program 2010;2010:153‐9. [PUBMED: 21239786] - PubMed
Macieji 2004 {published data only}
-
- Macieji K, Ina BK, Slawomir Z, Lukasz K. Use of small doses of recombinant factor VIIa during scoliosis surgery. Journal of Bone and Joint Surgery (Proceedings) 2004;86 B.C.(Suppl 1):94‐9a.
Nishijima 2009 {published data only}
-
- Nishijima DK, Zehtabchi S. Evidence‐based emergency medicine/critically appraised topic. The efficacy of recombinant activated factor VII in severe trauma. Annals of Emergency Medicine 2009;54(5):737‐44.e1. [PUBMED: 19285753] - PubMed
Perel 2010 {published data only}
Perez 2007 {published data only}
-
- Perez AF, Serra C, Macia J, Mayol L. rFVII for pediatric acute intracranial hemorrhage. Stroke 2007;38(7):E63‐4. - PubMed
Plaat 2007 {published data only}
-
- Plaat F. Recombinant factor VIIa should be used in massive obstetric haemorrhage. International Journal of Obstetric Anesthesia 2007;16(4):354‐7. - PubMed
Pugh 2007 {published data only}
-
- Pugh R, Wenstone R. Predicting response to recombinant factor VIIa in non‐haemophilia patients with severe haemorrhage (1). British Journal of Anaesthesia 2007;98(5):690. - PubMed
Strydom 2010 {published data only}
-
- Strydom J. The rational use of recombinant factor VIIa in the treatment of major intractable bleeding in the trauma patient. Southern African Journal of Anaesthesia and Analgesia 2010;16(1):130‐3.
Thabut 2011 {published data only}
-
- Thabut D, Rudler M, Rousseau G, Poynard T. Recombinant activated factor VII in chronic liver diseases: should we be afraid of thromboembolic events?. Journal of Hepatology 2011;55(2):483‐5. [PUBMED: 21349297] - PubMed
Van De Velde 2007 {published data only}
-
- Velde M. Recombinant factor VIIa should be used in massive obstetric haemorrhage. International Journal of Obstetric Anesthesia 2007;16(4):357‐9. - PubMed
Vincent 2009 {published data only}
-
- Vincent JL, Artigas A, Petersen LC, Meyer C. A multicenter, randomized, double‐blind, placebo‐controlled, dose‐escalation trial assessing safety and efficacy of active site inactivated recombinant factor VIIa in subjects with acute lung injury or acute respiratory distress syndrome. Critical Care Medicine 2009;37(6):1874‐80. - PubMed
Vink 2004 {published data only}
-
- Vink R, Bijsterveld NR, Aken BE, Fennema H, Peters RJG, Joost CM, et al. Recombinant factor VIIa reverses the anticoagulant effect of the long‐standing anticoagulant idraparinux in healthy volunteers. Blood 2004;102(11):812a. - PubMed
Woltz 2004 {published data only}
-
- Woltz M, Levi M, Sarich LC, Bostram SL, Eriksson UG, Eriksson‐Lepkoska M, et al. Effect of recombinant factor VIIa on melagatran‐induced inhibition of thrombin generation and platelet activation in healthy volunteers. Thrombosis and Haemostasis 2004;91(6):1090‐6. - PubMed
Yank 2009 {published data only}
-
- Yank V, Logan AC, Tuohy CV, Bravata DM, Staudenmayer K, Eisenhut R, et al. Comparison of thromboembolic event rates in randomized controlled trials and observational studies of recombinant factor VIIa for off‐label indications. A meta‐analysis. ASH Annual Meeting Abstracts. Blood 2009;114(22).
Yank 2011 {published data only}
Yuan 2010 {published data only}
-
- Yuan ZH, Jiang JK, Huang WD, Pan J, Zhu JY, Wang JZ. A meta‐analysis of the efficacy and safety of recombinant activated factor VII for patients with acute intracerebral hemorrhage without hemophilia. Journal of Clinical Neuroscience 2010;17(6):685‐93. [PUBMED: 20399668] - PubMed
References to ongoing studies
Arai 2005 {published and unpublished data}
-
- Arai M. Factor VIIa: Safety of Eptacog Alfa (activated) (genetical recombination) on adverse events and serious adverse events in patients with acute intracerebral haemorrhage. ClinicalTrials.gov 2005. [ClinicalTrials.gov Identifier: NCT00266006; Novo Nordisk Clinical Trial ID: F7ICH‐1602]
Flaherty 2008 {published data only}
-
- Flaherty ML, Jauch EC. The spot sign for predicting and treating intracerebral hemorrhage growth study. Clinicaltrials.gov 2008. [Clinicaltrials.gov Identifier: NCT00810888]
Gajewski 2005 {unpublished data only}
-
- Gajewski JL, Vogelsang G, Key N, Goodnough L. A multi‐center, randomized, double‐blind, parallel groups, placebo‐controlled trial on efficacy and safety of activated recombinant factor VII (rFVIIa/NovoSeven) in the treatment of bleeding in patients following hematopoietic stem cell transplantation (HSCT). Novo Nordisk Clinical Trial ID: F7SCT‐1485 2005.
Gladstone 2011 {published data only}
-
- Gladstone DJ, Aviv RI, Demchuk AM. "Spot sign" selection of intracerebral hemorrhage to guide hemostatic therapy (SPOTLIGHT). ClinicalTrials.gov 2010. [ClinicalTrials.gov identifier NCT01359202]
Gris 2006 {published data only}
-
- Gris JC. rhuFVIIa in post‐partum hemorrhage. ClinicalTrials.gov 2006. [Clinicaltrials.gov Identifier: NCT00370877]
Imberti 2005 {published and unpublished data}
-
- Imberti R. Efficacy and safety of factor VIIa (Eptacog Alfa) on rebleeding after surgery for spontaneous supratentorial intracerebral hemorrhage. A randomized, controlled, open‐label, investigator‐blinded pilot study. ClinicalTrials.gov 2005. [ClinicalTrials.gov Identifier: NCT00128050]
Iorio 2006 {published and unpublished data}
-
- Iorio A. Randomized, open, prospective, multicenter pilot study to evaluate the efficacy and safety of activated recombinant factor VII in acute intracerebral haemorrhage in patients treated with oral anticoagulants or antiplatelets agents. ClinicalTrials.gov 2006. [ClinicalTrials.gov Identifier: NCT00222625]
Kelleher 2006 {published data only}
-
- Kelleher A. A multi‐centre, randomised, double‐blind, placebo‐controlled, dose‐escalation trial of safety and efficacy of activated recombinant factor VII (Rfv11a/NovoSeven) in the treatment of post‐operative bleeding in patients following cardiac surgery requiring cardiopulmonary bypass. http://www2.rbht.nhs.uk/research/projects.
McCall 2005 {published and unpublished data}
-
- McCall P, Poustie S. "Salvage use" of recombinant activated factor VII after inadequate haemostatic response to conventional therapy in complex cardiac surgery ‐ a randomised placebo‐controlled trial. ClinicalTrials.gov 2005. [ClinicalTrials.gov Identifier: NCT00214656]
Molter 2005 {published and unpublished data}
-
- Molter NC, Park MS, Frail EA. Effect of recombinant coagulation factor VIIa on peri‐operative blood loss in patients undergoing major burn excision and grafting. ClinicalTrials.gov 2005. [ClinicalTrials.gov Identifier: NCT00243243]
Ng 2006 {published and unpublished data}
-
- Ng C. Use of recombinant activated factor seven (FVII) in bleeding ECMO patients post cardiac surgery. Randomised prospective study. National Research Register 2005. [N0012154243; ISRCTN98382551]
Additional references
Ahonen 2005
-
- Ahonen J, Jokela R. Recombinant factor VIIa for life‐threatening post‐partum haemorrhage. British Journal of Anaesthesia 2005;94(5):592‐5. - PubMed
Della Corte 2006
-
- Della Corte F. Efficacy and safety of factor VIIa on rebleeding after surgery for spontaneous intracerebral hemorhage (ICH). Clinical Trials.gov 2006. [Clinicaltrials.gov Identifier: NCT00128050]
Greisen 2003
-
- Greisen G, Andreasen RB. Recombinant factor VIIa in preterm neonates with prolonged prothrombin time. Blood Coagulation & Fibrinolysis 2003;14(1):117‐20. - PubMed
Hedner 2002
-
- Hedner U, Erhardtsen E. Potential role for rVIIa in transfusion medicine. Transfusion 2002;42(1):114‐24. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hsia 2008
-
- Hsia CC, Chin‐Yee IH, McAlister VC. Use of recombinant activated factor VII in patients without hemophilia: a meta‐analysis of randomized control trials. Annals of Surgery 2008;248(1):61‐8. - PubMed
Isbister 2008
-
- Isbister J, Phillips L, Dunkley S, Jankelowitz G, McNeil J, Cameron P. Recombinant activated factor VII in critical bleeding: experience from the Australian and New Zealand Haemostasis Register. Internal Medicine Journal 2008;38(3):156‐65. - PubMed
Key 2003a
-
- Key NS, Mast AE. Factor VIIa and tissue factor pathway inhibitor. In: Stowell C, Dzik W editor(s). Emerging Technologies in Transfusion Medicine. AABB Press, 2003.
Key 2003b
-
- Key NS. Recombinant FVIIa for intractable hemorrhage: more questions than answers. Transfusion 2003;43(12):1649‐51. - PubMed
Lipworth 2012
-
- Lipworth W, Kerridge I, Little M, Day R. Evidence and desperation in off‐label prescribing: recombinant factor VIIa. BMJ 2012;344:d7926. - PubMed
Logan 2011
Lusher 1998
-
- Lusher JM, Roberts HR, Davignon G, Joist JH, Smith H, Shapiro A, et al. A randomized, double‐blind comparison of two dosage levels of recombinant factor VIIa in the treatment of joint, muscle and mucocutaneous haemorrhages in persons with haemophilia A and B, with and without inhibitors. rFVIIa Study Group. Haemophilia 1998;4(6):790‐8. - PubMed
Marti‐Carvajal 2007
O'Connell 2006
-
- O'Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA 2006;295(3):293‐8. - PubMed
Ranucci 2008
-
- Ranucci M, Isgro G, Soro G, Conti D, Toffol B. Efficacy and safety of recombinant activated factor VII in major surgical procedures. Archives of Surgery 2008;143(3):296‐304. - PubMed
RevMan 2008 [Computer program]
-
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2008.
Roberts 2004
-
- Roberts HR, Monroe DM, White GC. The use of recombinant factor VIIa in the treatment of bleeding disorders. Blood 2004;104(13):3858‐64. - PubMed
References to other published versions of this review
Lin 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

