Calcineurin inhibitor minimisation versus continuation of calcineurin inhibitor treatment for liver transplant recipients
- PMID: 22419339
- PMCID: PMC11441157
- DOI: 10.1002/14651858.CD008852.pub2
Calcineurin inhibitor minimisation versus continuation of calcineurin inhibitor treatment for liver transplant recipients
Abstract
Background: The therapeutic success of liver transplantation has been largely attributable to the development of effective immunosuppressive treatment regimens. In particular, calcineurin inhibitors were essential in reducing acute rejection and improving early survival. Currently, more than 90% of all liver transplant recipients are treated with the calcineurin inhibitor cyclosporine or tacrolimus. Unfortunately, calcineurin inhibitors cause adverse events, such as nephrotoxicity, and because of this, minimisation (reduction and withdrawal) regimens of calcineurin inhibitor have been developed and studied. However, the benefits and harms of these minimisation regimens are unclear.
Objectives: To assess the benefits and harms of calcineurin inhibitor minimisation for liver transplant recipients without substitution by another immunosuppressive agent.
Search methods: We searched The Cochrane Hepato-Biliary Group Controlled Trials Register (Gluud 2010), Cochrane Central Register of Controlled Clinical Trials (CENTRAL) in The Cochrane Library, MEDLINE (OvidSP), EMBASE (OvidSP), Science Citation Index Expanded (Royle 2003), and the World Health Organization (WHO) international clinical trials registry platform (www.who.int/ictrp) until August 2011. In addition, we searched bibliographies of relevant articles as well as US Food and Drug Administration (FDA) and European Medicines Agency (EMA) drug approval reviews for additional trials.
Selection criteria: We planned to select all randomised clinical trials investigating calcineurin inhibitor reduction or withdrawal in liver transplant recipients, irrespective of blinding, publication status, or language. Quasi-randomised clinical studies and cohort studies that were obtained through the searches were considered only for the reporting of harms. Trials investigating substitution of one calcineurin inhibitor by another calcineurin inhibitor were excluded. Trials investigating calcineurin inhibitor withdrawal concurrently with switching over to a mammalian target of rapamycin (mTOR) inhibitor-based regimen (everolimus or sirolimus) or mycophenolate mofetil-based regimen are the subject of a separate review.
Data collection and analysis: Search strategies were used to obtain titles and abstracts of studies that were relevant for the review. Two authors independently scanned the references and assessed trial eligibility.
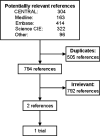
Main results: A total of 1299 references were identified by the searches. After removal of duplicates, 794 references were left. Out of these, two abstract reports of one ongoing randomised trial fulfilled the inclusion criteria of the review. This ongoing trial studies total withdrawal of immunosuppression in patients who receive a calcineurin inhibitor (cyclosporine or tacrolimus) or mycophenolate mofetil as the only immunosuppressive agent. The trial compares withdrawal of calcineurin inhibitor or mycophenolate mofetil with continuation of calcineurin inhibitor or mycophenolate mofetil. However, no trial results on the outcomes of interest to this review were available.
Authors' conclusions: This review shows that strategies regarding calcineurin inhibitor minimisation, that is, reduction or withdrawal, without substitution versus continuation of calcineurin inhibitor treatment lack evidence from randomised trials.More research with calcineurin inhibitor reduction and withdrawal regimens is needed to optimise dosing and timing of calcineurin inhibitor treatment in order to achieve optimal patient and graft survival with a minimum of adverse events.Specifically regarding calcineurin inhibitor reduction versus no reduction, we recommend that randomised trials evaluating calcineurin inhibitor reduction versus continuation of calcineurin inhibitor treatment are conducted.Regarding calcineurin inhibitor withdrawal, we recommend that mechanisms for tolerance and 'graft acceptance' are clarified, and patient groups likely to tolerate calcineurin inhibitor withdrawal are identified in order to select the right patients for total withdrawal of calcineurin inhibitors without substitution with another immunosuppressive drug. The randomised trials should only be performed in highly selected patients.
Conflict of interest statement
None known.
Figures
Update of
- doi: 10.1002/14651858.CD008852
References
References to studies excluded from this review
Assy 2007 {published data only}
-
- Assy N, Adams PC, Myers P, Simon V, Minuk GY, Wall W, et al. Randomized controlled trial of total immunosuppression withdrawal in liver transplant recipients: role of ursodeoxycholic acid. Transplantation 2007;83(12):1571‐6. - PubMed
Eason 2005 {published data only}
-
- Eason JD, Cohen AJ, Nair S, Alcantera T, Loss GE. Tolerance: is it worth the risk?. Transplantation 2005;79(9):1157‐9. - PubMed
Feng 2011 {published data only}
-
- Demetris AJ, Ruppert K, Ekong U, Lobritto SJ, Tchao NK, Feng S. Prospective evaluation of histopathology biopsy features that predict successful weaning and comparison to protocol follow‐up biopsies two years after complete immunosuppression withdrawal in living‐related pediatric liver allograft recipients. American Association for the Study of Liver Diseases (AASLD), Boston, USA. 2010.
-
- Feng S. Withdrawal of immunosuppression in pediatric liver transplant recipients (WISP‐R). http://clinicaltrials.gov 2011. [Clinicaltrials.gov NCT00320606]
-
- Feng S, Ekong U, Lobritto S, Demetris A, Rosenthal P, Alonso E, et al. [Clinical and histological predictors of operational tolerance in pediatric liver transplant recipients]. The 2011 Joint International Congress of ILTS, ELITA & LICAGE, Valencia, Spain. 2011.
Girlanda 2005 {published data only}
-
- Devlin J, Doherty D, Thomson L, Wong T, Donaldson P, Portmann B, et al. Defining the outcome of immunosuppression withdrawal after liver transplantation. Hepatology 1998;27:926‐33. - PubMed
-
- Girlanda R, Rela M, Williams R, O'Grady JG, Heaton ND. Long‐term outcome of immunosuppression withdrawal after liver transplantation. Transplantation Proceedings 2005;37(4):1708‐9. - PubMed
Mazariegos 1997 {published data only}
Orlando 2008 {published data only}
-
- Orlando G, Manzia T, Baiocchi L, Sanchez‐Fueyo A, Angelico M, Tisone G. The Tor Vergata weaning off immunosuppression protocol in stable HCV liver transplant patients: the updated follow up at 78 months. Transplant Immunology 2008;20:43‐7. - PubMed
-
- Tisone G, Orlando G, Cardillo A, Palmieri G, Manzia TM, Baiocchi L, et al. Complete weaning off immunosuppression in HCV liver transplant recipients is feasible and favourably impacts on the progression of disease recurrence. Journal of Hepatology 2006;44:702‐9. - PubMed
Pons 2009 {published data only}
-
- Pons JA, Ramirez P, Revilla‐Nuin B, Pascual D, Barajo‐Mazo A, Robles R, et al. Immunosuppression withdrawal improves long‐term metabolic parameters, cardiovascular risk factors and renal function in liver transplant patients. Clinical Transplantation 2009;23:329‐36. - PubMed
Sandborn 1994 {published data only}
-
- Sandborn WJ, Hay JE, Porayko MK, Gores GJ, Steers JL, Krom RA, et al. Cyclosporine withdrawal for nephrotoxicity in liver transplant recipients does not result in sustained improvement in kidney function and causes cellular and ductopenic rejection. Hepatology 1994;19(4):925‐32. - PubMed
Starzl 2003 {published data only}
Takatsuki 2001 {published data only}
-
- Takatsuki M, Uemoto S, Inomata Y, Egawa H, Kiuchi T, Fujita S, et al. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation 2001;72(3):449‐54. - PubMed
Thistlewaith 2011 {published data only}
-
- Thistlewaith JR. Safety and efficacy of campath‐1H and tacrolimus followed by immunosuppression withdrawal in liver transplantation. www.immunetolerance.org 2011. [www.immunetolerance.org ITN024ST]
Tryphonopoulus 2010 {published data only}
-
- Tryphonopoulus P, Ruiz P, Weppler D, Nishida S, Levi DM, Moon J, et al. Long‐term follow‐up of 23 operational tolerant liver transplant recipients. Transplantation 2010;90:1556‐61. - PubMed
References to ongoing studies
Shaked 2011 {published data only}
-
- Feng S, Punch J, Reyes J, Levitsky J, Klintmalm G, Zimmerman M, et al. Evolution of donor specific antibodies with immunosuppression withdrawal among adult liver transplant recipients in ITN030ST. The 2011 Joint International Congress of ILTS, ELITA & LICAGE, Valencia, Spain. 2011.
-
- Shaked A, Feng S, Punch J, Reyes, J, Levitsky J, Klintmalm G, et al. [Lessons learned form the ITN030ST immunosuppression withdrawal trial in liver transplant recipients]. The 2011 Joint International Congress of ILTS, ELITA & LICAGE, Valencia, Spain. 2011.
-
- Shaked A, et al. Gradual withdrawal of immune system suppressing drugs in patients receiving a liver transplant (AWISH). http://clinicaltrials.gov. [clinicaltrials.gov NCT 00135694; www.immunetolerance.org ITN030ST]
Additional references
Altman 2003
Chatenoud 2008
-
- Chatenoud L. The long and winding road towards induction of allograft tolerance in the clinic. Transplant International 2008;21(8):725‐7. - PubMed
Dantal 2005
-
- Dantal J, Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. New England Journal of Medicine 2005;352:1371‐3. - PubMed
Egger 1997
ELTR 2010
-
- European Liver Transplant Registry. www.eltr.org (accessed 9 December 2011).
Farkas 2009
-
- Farkas SA, Schnitzbauer AA, Kirchner G, Obed A, Banas B, Schlitt HJ. Calcineurin inhibitor minimization protocols in liver transplantation. Transplant International 2009;22:49‐60. - PubMed
Flechner 2008
-
- Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor‐sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clinical Transplantation 2008;22(1):1‐15. - PubMed
Gluud 2006
-
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology 2006;163(6):493‐501. - PubMed
Gluud 2011
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2011, Issue 12. Art. No.: LIVER.
Hegarty 1983
-
- Hegarty JE, Nouri Aria KT, Portmann B, Eddleston AL, Williams R. Relapse following treatment withdrawal in patients with autoimmune chronic active hepatitis. Hepatology 1983;3(5):685‐9. - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Colloboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Lechler 2005
-
- Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation ‐ how much of the promise has been realized?. Nature Medicine 2005;11(6):605‐13. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
McAlister 2006
-
- McAlister VC, Haddad E, Renouf E, Malthaner RA, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta‐analysis. American Journal of Transplantation 2006;6(7):1578‐85. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. - PubMed
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. - PubMed
Ojo 2003
-
- Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. New England Journal of Medicine 2003;349:931‐40. - PubMed
OPTN 2008
-
- OPTN / SRTR Annual Report 2008. The U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. www.optn.transplant.hrsa.gov (accessed December 2011).
Pawelec 1995
-
- Pawelec G, Adibzadeh M, Pohla H, Schaudt K. Immunosenescence: ageing of the immune system. Immunology Today 1995;16:420‐2. - PubMed
Penninga 2010
Perera 2009
-
- Perera MT, Mirza DF, Elias E. Liver transplantation: Issues for the next 20 years. Journal of Gastroenterology and Hepatology 2009;24 Suppl 3:124‐31. - PubMed
Pillai 2009
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Seyfert‐Margolis 2010
-
- Seyfert‐Margolis V, Feng S. Tolerance: is it achievable in pediatric solid organ transplantation?. Pediatric Clinics of North America 2010;57:523‐38. - PubMed
Starzl 1981
Starzl 1992
Starzl 2010
Thompson 2002
-
- Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21(11):1559‐73. - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
Wiesner 2011
-
- Wiesner RH, Fung JJ. Present state of immunosuppressive therapy in liver transplant recipients. Liver Transplantation 2011;17 Suppl:1‐9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous