Micronutrient supplementation in pregnant women with HIV infection
- PMID: 22419344
- PMCID: PMC11747962
- DOI: 10.1002/14651858.CD009755
Micronutrient supplementation in pregnant women with HIV infection
Abstract
Background: Micronutrient deficiencies are widespread and compound the effects of HIV disease; micronutrient supplements may be effective and safe in reducing this burden.
Objectives: To assess whether micronutrient supplements are effective and safe in reducing mortality and morbidity in pregnant and lactating women with HIV infection and their infants.
Search methods: The review has been updated three times since publication in 2005. In reviews prior to this update (2011), we searched the CENTRAL, EMBASE, PubMed, and GATEWAY databases to identify randomised controlled trials of micronutrient supplements using the search methods of the Cochrane HIV/AIDS Group. In the 2011 review the PubMed, EMBASE, and CENTRAL databases were searched in July 2011. As the GATEWAY database does not include conference abstracts after 2006, we also searched the AIDS-specific conference database, www.aegis.org, and contacted researchers and organisations active in the field of research to identify additional unpublished trials.
Selection criteria: Randomised controlled trials were selected that compared the effects of micronutrient supplements (vitamins, trace elements, and combinations of these) with other supplements, placebo or no treatment on mortality, morbidity, pregnancy outcomes, immunologic indicators, and anthropometric measures in HIV-positive pregnant and lactating women. Any adverse effects of supplementation were recorded.
Data collection and analysis: Two reviewer authors independently selected trials, appraised trial quality for risk of bias using standardised criteria, and extracted data using standardised forms. Where disagreements arose, a third author, acted as arbiter.
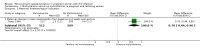
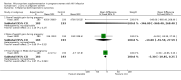
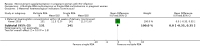
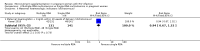
Main results: One additional trial is included in this update in addition to the three trials included in the 2010 update of the initial Cochrane review. Four relatively large, well-conducted randomised controlled trials of the benefits of micronutrient supplementation have been conducted in pregnant and lactating women infected with HIV. Each of the trials evaluated a different micronutrient supplement and no direct comparisons or analyses can be made across the four trials. The four trials were conducted between 1995 and 2006. The trials have all been conducted by the same research team in Dar es Salaam in Tanzania, in an urban setting in hospital-based antenatal clinics. Pregnant women were recruited with gestational age ranging from 12 to 27 weeks in each of the trials. Sample sizes range from 400 to 1129 with a median of 1000 participants. Three of the trials were placebo-controlled. Different interventions have been evaluated in each trial, viz.:Vitamin A versus Vitamin A and multivitamins versus Multivitamins versus placebo; Selenium versus placebo; Zinc versus placebo; and Multiple RDA multivitamins versus Single RDA multivitamins. None of the women were receiving antiretrovrial therapy (ART).Multiple micronutrient supplements conferred multiple clinical benefits to pregnant women and their offspring. No significant adverse effects were reported.No significant clinical benefits were found from zinc supplementation of pregnant Tanzanian women.Selenium supplements given during and after pregnancy did not delay maternal HIV disease progression or improve pregnancy outcomes, but may improve child survival and decrease maternal diarrhoeal morbidity.There were no differences in maternal and infant outcomes when women received single RDA multivitamins or multiple RDA multivitamin supplementation.The evidence is lacking for the effects of micronutrient supplementation given concomitantly to pregnant women already initiated on antiretroviral therapy for treatment purposes.GRADE assessments were conducted on outcomes for each trial and included reviewing the data and the potential biases in each trial before grading the level of evidence. None of the trials were graded as providing high quality evidence primarily because there was no replication of results in other trials in other settings.
Authors' conclusions: In keeping with previous World Health Organization (WHO) recommendations everything possible should be done to promote and support adequate dietary intake of micronutrients, while recognising that this may not be sufficient to correct specific micronutrient deficiencies in all HIV-infected individuals.Specific recommendations for pregnant and lactating women infected with HIV would be to include the provision of multivitamin supplements in single RDA formulations during the antenatal period and at least for 6 weeks post-partum, especially for women who are breast-feeding.There is no conclusive evidence to provide stand-alone zinc or selenium supplementation to HIV-infected pregnant and lactating women.Micronutrient supplementation should not be used as a substitute for provision of recommended antiretroviral medication for preventing mother-to-child transmission of HIV and treating maternal HIV infection when this is recommended.Further trials of single supplements are required to build the evidence base. The long-term clinical benefits, adverse effects, and optimal formulation of multiple micronutrient supplements require further investigation in pregnant women at different stages of HIV infection.
Conflict of interest statement
None.
Figures























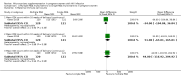

References
References to studies included in this review
Fawzi 1998 {published data only}
-
- Baylin A, Villamor E, Rifai N, Msamanga G, Fawzi WW. Effect of vitamin supplementation to HIV‐infected pregnant women on the micronutrient status of their infants. European journal of clinical nutrition 2005;59(8):960‐8. - PubMed
-
- Fawzi WW, Msamanga GI, Hunter D, Renjifo B, Antelman G, Bang H, et al. Randomized trial of vitamin supplements in relation to transmission of HIV‐1 through breastfeeding and early child mortality. AIDS 2002;16(14):1935‐42. - PubMed
-
- Fawzi WW, Msamanga GI, Hunter D, Urassa E, Renjifo B, Mwakagile D, et al. Randomized trial of vitamin supplements in relation to vertical transmission of HIV‐1 in Tanzania. Journal of acquired immune deficiency syndromes (1999) 2000;23:246‐54. - PubMed
-
- Fawzi WW, Msamanga GI, Spiegelman D, Urassa E, McGrath N, Mwakagile D, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV‐1‐infected women in Tanzania. Lancet 1998;351:1477‐82. - PubMed
-
- Fawzi WW, Msamanga GI, Spiegelman D, Wei R, Kapiga S, Villamor E, et al. A randomized trial of multivitamin supplements and HIV disease progression and mortality. The New England journal of medicine 2004;351(1):23‐32. - PubMed
Fawzi 2005 {published data only}
-
- Fawzi WW, Villamor E, Msamanga GI, Antelman G, Aboud S, Urassa W, et al. Trial of zinc supplements in relation to pregnancy outcomes, hematologic indicators, and T cell counts among HIV‐1‐infected women in Tanzania. The American journal of clinical nutrition 2005;81(1):161‐7. - PubMed
-
- Villamor E, Aboud S, Koulinska IN, Kupka R, Urassa W, Chaplin B, et al. Zinc supplementation to HIV‐1‐infected pregnant women: Effects on maternal anthropometry, viral load, and early mother‐to‐child transmission. European journal of clinical nutrition 2006;60:862‐9. - PubMed
Kawai 2010 {published data only}
-
- Kawai K, Kupka R, Mugusi F, Aboud S, Okuma J, Villamor E, et al. A randomized trial to determine the optimal dosage of multivitamin supplements to reduce adverse pregnancy outcomes among HIV‐infected women in Tanzania. The American journal of clinical nutrition 2010;91(2):391‐7. [PUBMED: 19939985] - PMC - PubMed
Kupka 2008 {published data only}
-
- Kupka R, Mugusi F, Aboud S, Msamanga GI, Finkelstein JL, Spiegelman D. Randomized, double‐blind, placebo‐controlled trial of selenium supplements among HIV‐infected pregnant women in Tanzania: effects on maternal and child outcomes. The American journal of clinical nutrition 2008;87:1802‐8. - PMC - PubMed
References to studies excluded from this review
Austin 2003 {published data only}
-
- Austin J, Voigt R, Smaill F, Gill J, Walmsley S, Gilmour J, et al. A randomised controlled trial of daily multivitamin with or without mixed carotenoid supplementtation in advanced HIV disease. International Conference on AIDS. 2000.
Constans 1996 {published data only}
-
- Constans J, Delmas‐Beauvieux MC, Sergeant C, Peuchant E, Pellegrin JL, Pellegrin I, et al. One‐year antioxidant supplementation with beta‐carotene or selenium for patients infected with human immunodeficiency virus: a pilot study. Clinical infectious diseases 1996; Vol. 23, issue 3:654‐6. - PubMed
Kennedy 2000 {published data only}
-
- Kennedy CM, Coutsoudis A, Kuhn L, PIllay K, Mburu A, Stein Z, et al. Randomized controlled trial assessing the effect of vitamin A supplementation on maternal morbidity during pregnancy and postpartum among HIV‐infected women. Journal of acquired immune deficiency syndromes 2000;24(1):37‐44. - PubMed
Kumwenda 2002 {published data only}
-
- Kumwenda N, Miotti PG, Taha TE, Broadhead R, Biggar RJ, Jackson JB, et al. Antenatal vitamin A supplementation increases birth weight and decreases anemia among infants born to human immunodeficiency virus‐infected women in Malawi. Clinical infectious diseases 2002;35(5):618‐24. - PubMed
Mehta 2009 {published data only}
-
- Mehta S, Hunter DJ, Mugusi FM, Speigelman D, Manji KP, Giovannucci EL, et al. Perinatal outcomes, including mother‐to‐child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. The Journal of infectious diseases 2009;200(7):1022‐30. - PMC - PubMed
Shor‐Posner 2003 {published data only}
-
- Shor‐Posner G, Lecusay R, Miguez MJ, Moreno‐Black G, Zhang G, Rodriguez N, et al. Psychological burden in the era of HAART: impact of selenium therapy. International journal of psychiatry in medicine 2003;33(1):55‐69. - PubMed
References to studies awaiting assessment
Kindra 2011 {published data only}
Matovu 2002 {unpublished data only}
-
- Matovu SM, Francis KF, Martin OM, Mayanja James MJ. Nutrition in HIV Transmission and Management. The AIDS Support Organisation (TAS0)(U)Ltd, Kampala, Uganda. Int Conf AIDS. 2002; Vol. Jul 7 ‐ 12:14:(abstract no. B10485).
Additional references
Black 2008
-
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243‐60. [PUBMED: 18207566] - PubMed
Chan 2005
-
- Chan AW, Altman DG. Epidemiology and reporting of randomised trials published in PubMed journals. Lancet 2005;365(9465):1159‐62. [PUBMED: 15794971] - PubMed
Christian 2003
-
- Christian P, Shrestha J, LeClerq SC, Khatry SK, Jiang T, Wagner T, et al. Supplementation with micronutrients in addition to iron and folic acid does not further improve the hematologic status of pregnant women in rural Nepal. The Journal of nutrition 2003;133(11):3492‐8. [PUBMED: 14608063] - PubMed
Christian 2007
-
- Christian P, West KP. Technical Brief: Prenatal multiple micro‐nutrient supplementation: current state of knowledge and priorities. http://a2zproject.org/˜a2zorg/pdf/AntenatalMM.pdf Accessed on::20 December 2011.
Churchill 1989
-
- R. Koenigsberg, ed. Churchill's Illustrated Medical Dictionary. New York: Churchill‐Livingstone Inc, 1989.
de Pee 2010
-
- Pee S, Semba RD. Role of nutrition in HIV infection: review of evidence for more effective programming in resource‐limited settings. Food and nutrition bulletin 2010;13(4):S313‐44. - PubMed
Fawzi 1999
-
- Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, Hunter DJ. Rationale and design of the Tanzania Vitamin and HIV Infection Trial. Controlled clinical trials 1999;20(1):75‐90. [PUBMED: 10027501] - PubMed
Fawzi 2003
-
- Fawzi WW, Msamanga GI, Wei R, Spiegelman D, Antelman G, Villamor E, et al. Effect of providing vitamin supplements to human immunodeficiency virus‐infected, lactating mothers on the child's morbidity and CD4+ cell counts. Clinical infectious diseases 2003;36:1053‐62. - PubMed
Fawzi 2004
-
- Fawzi WW, Msamanga GI, Spiegelman D, Wei R, Kapiga S, Villamor E, et al. A randomized trial of multivitamin supplements and HIV disease progression and mortality. The New England journal of medicine 2004;351(1):23‐32. - PubMed
Forrester 2011
GRADEpro [Computer program]
-
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. [Computer program]. Version 3.2 for Windows.. The GRADE Working Group, 2008.
Guyatt 2011
-
- Guyatt, G, Oxman, A.D, Akl, E.A, Kunz, R, Vist, G, Brozek, J, Norris, S, Falck‐Ytter, Y, Glasziou, P, DeBeer, H, Jaeschke, R, Rind, D, Meerpohl, J, Dahm, P, Schunemann. H.J. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383‐94. - PubMed
Haider 2006
-
- Haider BA, Bhutta ZA. Multiple‐micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 4. [PUBMED: 17054223] - PubMed
Haider 2011
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Irlam 2010
Mehta 2010
Merchant 2005
-
- Merchant AT, Msamanga G, Villamor E, Saathoff E, O'brien M, Hertzmark E, et al. Multivitamin supplementation of HIV‐positive women during pregnancy reduces hypertension. The Journal of nutrition 2005;135(7):1776‐81. - PubMed
Micronutrient Initiative 2009
-
- The Micronutrient Initiative. Investing in the Future: A United Call to Action on Vitamin and Mineral Deficiencies Global Report 2009. http://www.unitedcalltoaction.org/documents/Investing_in_the_future (accessed 13 August 2011).
Overton 2011
-
- Overton ET, Yin MT. The rapidly evolving research on vitamin D among HIV‐infected populations. Current infectious disease reports 2011;13(1):83‐93. [PUBMED: 21308459] - PubMed
Owens 2008
Ramakrishnan 2004
-
- Ramakrishnan U, Neufeld LM, Gonzalez‐Cossio T, Villalpando S, Garcia‐Guerra A, Rivera J, et al. Multiple micronutrient supplements during pregnancy do not reduce anemia or improve iron status compared to iron‐only supplements in Semirural Mexico. The Journal of nutrition 2004;134(4):898‐903. [PUBMED: 15051844] - PubMed
Schneider 2008
-
- Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, McKenna MT. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years‐‐United States, 2008. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control 2008;57(RR‐10):1‐12. - PubMed
Smith 2007
UNICEF 2009
-
- Tracking progress on child and maternal nutrition. A survival and development priority. United Nations Children's Fund (UNICEF), 2009:124.
van den Broek 2010
-
- Broek N, Dou L, Othman M, Neilson JP, Gates S, Gulmezoglu AM. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database of Systematic Reviews 2010, Issue 11. [PUBMED: 21069707] - PubMed
Villamor 2002
-
- Villamor E, Msamanga GI, Spiegelman D, Antelman G, Peterson KE, Hunter DJ, et al. Effect of multivitamin and vitamin A supplements on weight gain during pregnancy among HIV‐1‐infected women. The American journal of clinical nutrition 2002;76(5):1082‐90. - PubMed
Villamor 2005
-
- Villamor E, Saathoff E, Bosch RJ, Hertzmark E, Baylin A, Manji K, et al. Vitamin supplementation of HIV‐infected women improves postnatal child growth. The American journal of clinical nutrition 2005;81(4):880‐8. - PubMed
WHO 2003
-
- World Health Organization. Nutrient requirements for people living with HIV/AIDS: report of a technical consultation. World Health Organization, Geneva 2003.
WHO 2007
-
- World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV‐related diseases in adults and children. World Health Organization, Geneva 2007.
WHO 2010
-
- World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. World Health Organization, Geneva 2010. - PubMed
WHO/UNICEF 2004
-
- Clinical Management of Acute Diarrhoea: Joint Statement 2004. WHO/UNICEF.
References to other published versions of this review
Irlam 2005
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

