Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection
- PMID: 22419345
- PMCID: PMC6486190
- DOI: 10.1002/14651858.CD009756
Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection
Abstract
Background: More than 34 million people are presently living with HIV infection. Antiretroviral therapy (ART) can help these people to live longer, healthier lives, but adherence to ART can be difficult. Mobile phone text-messaging has the potential to help promote adherence in these patients.
Objectives: To determine whether mobile phone text-messaging is efficacious in enhancing adherence to ART in patients with HIV infection.
Search methods: Using the Cochrane Collaboration's validated search strategies for identifying randomised controlled trials and reports of HIV interventions, along with appropriate keywords and MeSH terms, we searched a range of electronic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, Literatura Latino-Americana e do Caribe em Ciências da Saúde (LILACS), MEDLINE (via PubMed), PsycINFO, Web of Science, and the World Health Organization (WHO) Global Index Medicus. The date range was from 01 January 1980 to 01 November 2011. There were no limits to language or publication status.
Selection criteria: Randomised controlled trials (RCTs) in which patients or their caregivers (in the case of infants and children) of any age, in any setting, and receiving ART were provided with mobile phone text messages as a means of promoting adherence to ART.
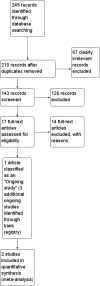
Data collection and analysis: Two authors independently examined the abstracts of all identified trials. We initially identified 243 references. Seventeen full-text articles were closely reviewed. Both authors abstracted data independently, using a pre-designed, standardised data collection form. When appropriate, data were combined in meta-analysis.
Main results: Two RCTs from Kenya were included in the review. One trial compared short weekly text messages against standard care. The other trial compared short daily, long daily, short weekly and long weekly messages against standard care. Both trials were with adult patients.In the trial comparing only short weekly messages to standard care, text messaging was associated with a lower risk of non-adherence at 12 months (RR 0.77, 95% CI 0.63 to 0.93) and with the non-occurrence of virologic failure at 12 months (RR 0.83, 95% CI 0.69 to 0.99).In the trial that compared different intervals and lengths for text-messaging to standard care, long weekly text-messaging was not significantly associated with a lower risk of non-adherence compared to standard care (RR 0.79, 95% CI 0.60 to 1.04). Patients receiving weekly text-messages of any length were at lower risk of non-adherence at 48 weeks than were patients receiving daily messages of any length (RR 0.79, 95% CI 0.64 to 0.99). There were no significant differences between weekly text-messaging of any length (RR 1.01, 95% CI 0.75 to 1.37) and between short or long messaging at either interval (RR 0.99, 95% CI 0.78 to 1.27). Compared to standard care, any daily text-messaging, whether short or long, did not reduce the risk for non-adherence (RR 0.99, 95% CI 0.82 to 1.20).In meta-analysis of both trials, any weekly text-messaging (i.e. whether short or long messages) was associated with a lower risk of non-adherence at 48-52 weeks (RR 0.78, 95% CI 0.68 to 0.89). The effect of short weekly text-messaging was also significant (RR 0.77, 95% CI 0.67 to 0.89).
Authors' conclusions: There is high-quality evidence from the two RCTs that mobile phone text-messaging at weekly intervals is efficacious in enhancing adherence to ART, compared to standard care. There is high quality evidence from one trial that weekly mobile phone text-messaging is efficacious in improving HIV viral load suppression. Policy-makers should consider funding programs proposing to provide weekly mobile phone text-messaging as a means for promoting adherence to antiretroviral therapy. Clinics and hospitals should consider implementing such programs. There is a need for large RCTs of this intervention in adolescent populations, as well as in high-income countries.
Conflict of interest statement
No known conflicts of interest.
Figures

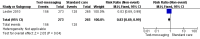
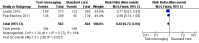
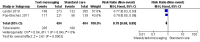
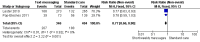
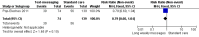
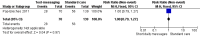
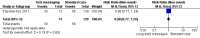
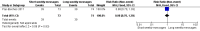
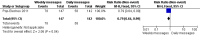












References
References to studies included in this review
Lester 2010 {published data only}
-
- Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet 2010 Nov 27;376(9755):1838‐45. - PubMed
Pop‐Eleches 2011 {published data only}
References to studies excluded from this review
Ammassari 2011 {published data only}
-
- Ammassari A, Trotta MP, Shalev N, Tettoni MC, Maschi S, Sora F, et al. Timed short messaging service improves adherence and virological outcomes in HIV‐1‐infected patients with suboptimal adherence to antiretroviral therapy. Journal of acquired immune deficiency syndromes (1999) 2011; Vol. 58, issue 4:e113‐5. [PUBMED: 22033235] - PubMed
Chi 2010 {published data only}
-
- Chi BH, Stringer JS. Mobile phones to improve HIV treatment adherence. Lancet 2010 Nov 27;376(9755):1807‐8. - PubMed
De Costa 2010 {published data only}
-
- Costa A, Shet A, Kumarasamy N, Ashorn P, Eriksson B, Bogg L, et al. Design of a randomized trial to evaluate the influence of mobile phone reminders on adherence to first line antiretroviral treatment in South India‐‐the HIVIND study protocol. BMC medical research methodology 2010;10:25. [PUBMED: 20346136] - PMC - PubMed
Dowshen 2011 {published data only}
-
- Dowshen N, Kuhns L, Johnson A, Holoyda B, Garofalo R. Text Message Reminders to Improve Adherence to Antiretroviral Therapy for HIV‐Positive Youth. Journal of Adolescent Health Feb 2011; Vol. Volume 48, Issue 2, Supplement 1:S64‐S65.
Haberer 2010 {published data only}
Hardy 2011 {published data only}
Kalichman 2011 {published data only}
Kelly 2011 {published data only}
-
- Kelly JD, Giordano TP. Mobile phone technologies improve adherence to antiretroviral treatment in a resource‐limited setting: a randomized controlled trial of text message reminders. AIDS 2011 May 15;25(8):1137; reply 8‐9. - PubMed
Mukund Bahadur 2010 {published data only}
-
- Mukund Bahadur KC, Murray PJ. Cell phone short messaging service (SMS) for HIV/AIDS in South Africa: a literature review. Stud Health Technol Inform 210;160:Pt 1:530‐534. - PubMed
Puccio 2006 {published data only}
-
- Puccio JA, Belzer M, Olson J, Martinez M, Salata C, Tucker D, et al. The use of cell phone reminder calls for assisting HIV‐infected adolescents and young adults to adhere to highly active antiretroviral therapy: a pilot study. AIDS Patient Care STDS 2006 Jun;20(6):438‐44. - PubMed
Reynolds 2008 {published data only}
-
- Reynolds NR, Testa MA, Su M, Chesney MA, Neidig JL, Frank I, et al. Telephone support to improve antiretroviral medication adherence: a multisite, randomized controlled trial. J Acquir Immune Defic Syndr 2008 Jan 1;47(1):62‐8. - PubMed
Saberi 2011 {published data only}
Safren 2003 {published data only}
-
- Safren SA, Hendriksen ES, Desousa N, Boswell SL, Mayer KH. Use of an on‐line pager system to increase adherence to antiretroviral medications. AIDS Care 2003 Dec;15(6):787‐93. - PubMed
Simoni 2007 {published data only}
Skinner 2007 {published data only}
-
- Skinner D, Rivette U, Bloomberg C. Evaluation of use of cellphones to aid compliance with drug therapy for HIV patients. AIDS Care 2007 May;19(5):605‐7. - PubMed
Wang 2008 {published data only}
-
- Wang H, He G, Li X, Yang A, Chen X, Fennie KP, et al. Self‐Reported adherence to antiretroviral treatment among HIV‐infected people in Central China. AIDS Patient Care STDS 2008 Jan;22(1):71‐80. - PubMed
References to studies awaiting assessment
da Costa 2012 {published data only}
-
- Costa TM, Barbosa BJ, E Costa DA, Sigulem D, Fatima Marin H, Filho AC, et al. Results of a randomized controlled trial to assess the effects of a mobile SMS‐based intervention on treatment adherence in HIV/AIDS‐infected Brazilian women and impressions and satisfaction with respect to incoming messages. International journal of medical informatics 2012 Jan 30;[Epub ahead of print]:n/a. [PUBMED: 22296762] - PMC - PubMed
References to ongoing studies
Mbuagbaw 2011 {published data only}
-
- Mbuagbaw L, Thabane L, Ongolo‐Zogo P, Lester RT, Mills E, Volmink J, Yondo D, Essi MJ, Bonono‐Momnougui RC, Mba R, Ndongo JS, Nkoa FC, Ondoa HA. The Cameroon mobile phone SMS (CAMPS) trial: a protocol for a randomized controlled trial of mobile phone text messaging versus usual care for improving adherence to highly active anti‐retroviral therapy. Trials 2011 Jan 7;12:5. PubMed PMID: 21211064;PubMed Central PMCID:PMC3023676. - PMC - PubMed
NCT01001741 {published data only}
-
- A Pilot Study of Cellular Phone Text Message Reminders to Improve HIV Medication Adherence at Independence Surgery Clinic Gaborone, Botswana. Ongoing study 2008.
NCT01049568 {published data only}
-
- A Pilot Study Using Cell Phone Interactions to Improve Medication Adherence in Adolescents Who Have Previously Failed Antiretroviral Therapy Due to Non‐Adherence. Ongoing study 2009.
NCT01118767 {published data only}
-
- Evaluation of a Computer‐Based System Using Cell Phones for HIV People in Peru. Ongoing study 2010.
Additional references
Anglemyer 2011
Atun 2006
-
- Atun RA, Sittampalam S. A review of the characteristics and benefits of SMS in delivering healthcare. In: Atun RA, et al. editor(s). The role of mobile phones in increasing accessibility and efficiency in healthcare. Vodafone Group Plc, 2006.
Bain‐Brickley 2011
Barnighausen 2011
Chang 2011
GRADEpro 2008 [Computer program]
-
- Brozek J, Oxman A, Schünemann. GRADEpro. GRADE Working Group, 2008.
Guardian 2009
-
- Wray R. Africa sees massive growth in mobile web usage. The Guardian, 2009. Available from: http://www.guardian.co.uk/technology/2009/dec/22/mobilephones‐internet.
Guyatt 2008
Higgins 2008
-
- Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley‐Blackwell, 2008.
iTWire 2011
-
- Anonymous. Big three fuel Asia Pacific Mobile Growth. Available from: http://www.itwire.com/it‐industry‐news/market/49228‐big‐three‐fuel‐asia‐....
Leong 2006
-
- Leong KC, Chen WS, Leong KW, Mastura I, Mimi O, Sheikh MA, Zailinawati AH, Ng CJ, Phua KL, Teng CL. The use of text messaging to improve attendance in primary care: a randomized controlled trial. Fam Pract 2006;23:699‐705. - PubMed
Liu 2008
-
- Liu Q, Abba K, Alejandria Marissa M, Balanag Vincent M, Berba Regina P, Lansang Mary Ann D. Reminder systems and late patient tracers in the diagnosis and management of tuberculosis. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006594.pub2; CD006594] - DOI - PubMed
Menon‐Johansson 2006
Mills 2006
Palella 1998
-
- Palella FJ, Delaney KM, Moorman AC, Loveless MO, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998 Mar 26;338(13):853‐60. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan), Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rueda 2006
-
- Rueda S, Park‐Wyllie Laura Y, Bayoumi A, Tynan A‐M, Antoniou T, Rourke S, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001442.pub2; CD001442] - DOI - PMC - PubMed
UNAIDS 2011
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS), World Health Organization (WHO), United Nations Children's Fund (UNICEF). Global HIV/AIDS Response: Epidemic update and health sector progress towards Universal Access. Geneva: UNAIDS, 2011.
WHO 2009
-
- World Health Organization. Millennium Development Goal 6: Combat HIV/AIDS, malaria, and other diseases. Available from: http://www.who.int/hiv/topics/mdg/info/en/.
WHO 2010
-
- World Health Organization. HIV drug resistance fact sheet. Available from: http://www.who.int/hiv/facts/drug_resistance/en/index.html.
Wise 2008
-
- Wise J, Operario D. Use of electronic reminder devices to improve adherence to antiretroviral therapy: a systematic review. AIDS patient care and STDs 2008;22(6):495‐504. [PUBMED: 18462071] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

