Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition
- PMID: 22419721
- PMCID: PMC3339884
- DOI: 10.1210/jc.2011-3251
Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition
Abstract
Context: Progesterone promotes uterine relaxation during pregnancy and its withdrawal induces labor. Progesterone withdrawal in human parturition is mediated in part by changes in the relative levels of the nuclear progesterone receptor isoforms, PR-A and PR-B, in myometrial cells. Parturition also involves myometrial inflammation; however, the functional link between nuclear PR-mediated progesterone actions and inflammation in human myometrial cells is unclear.
Objective: Our objective was to determine how PR-A and PR-B regulate progesterone action in human myometrial cells and specifically the expression of genes encoding contraction-associated proteins and proinflammatory mediators.
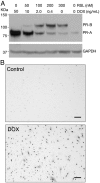
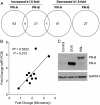
Design: Effects of PR-A and PR-B on the capacity for progesterone to modulate gene expression was determined using an immortalized human myometrial cell line stably transfected with inducible PR-A and PR-B expression transgenes and conditioned to express various PR-A and PR-B levels. Gene expression was assessed by genome wide transcriptome analysis, quantitative RT-PCR and immunoblotting.
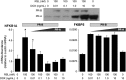
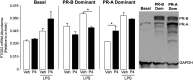
Results: PR-A and PR-B were each transcriptionally active in response to progesterone and affected the expression of distinct gene cohorts. The capacity for progesterone to affect gene expression was dependent on the PR-A to PR-B ratio. This was especially apparent for the expression of proinflammatory genes. Progesterone decreased proinflammatory gene expression when the PR-A to PR-B ratio favored PR-B and increased proinflammatory gene expression when the ratio favored PR-A. Progesterone via PR-B increased expression of inhibitor-κBα, a repressor of the nuclear factor-κB transcription factor, and inhibited basal and lipopolysaccharide-induced proinflammatory gene expression. Both of those PR-B-mediated effects were inhibited by PR-A.
Conclusions: Our data suggest that during most of human pregnancy, when myometrial cells are PR-B dominant, progesterone promotes myometrial quiescence through PR-B-mediated antiinflammatory actions. At parturition, the rise in PR-A expression promotes labor by inhibiting the antiinflammatory actions of PR-B and stimulating proinflammatory gene expression in response to progesterone.
Figures

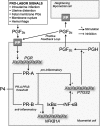
References
-
- Corner GW. 1946. The hormones in human reproduction. London: Princeton University Press
-
- Csapo A. 1956. Progesterone block. Am J Anat 98:273–291 - PubMed
-
- Sanborn BM. 2000. Relationship of ion channel activity to control of myometrial calcium. J Soc Gynecol Investig 7:4–11 - PubMed
-
- Sanborn BM, Yue C, Wang W, Dodge KL. 1998. G protein signalling pathways in myometrium: affecting the balance between contraction and relaxation. Rev Reprod 3:196–205 - PubMed
-
- Sanborn BM. 1995. Ion channels and the control of myometrial electrical activity. Semin Perinatol 19:31–40 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

