Constitutive gray hair in mice induced by melanocyte-specific deletion of c-Myc
- PMID: 22420299
- PMCID: PMC3541053
- DOI: 10.1111/j.1755-148X.2012.00998.x
Constitutive gray hair in mice induced by melanocyte-specific deletion of c-Myc
Abstract
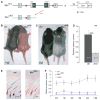
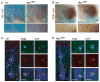
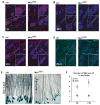
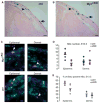
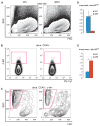
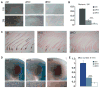
c-Myc is involved in the control of diverse cellular processes and implicated in the maintenance of different tissues including the neural crest. Here, we report that c-Myc is particularly important for pigment cell development and homeostasis. Targeting c-Myc specifically in the melanocyte lineage using the floxed allele of c-Myc and Tyr::Cre transgenic mice results in a congenital gray hair phenotype. The gray coat color is associated with a reduced number of functional melanocytes in the hair bulb and melanocyte stem cells in the hair bulge. Importantly, the gray phenotype does not progress with time, suggesting that maintenance of the melanocyte through the hair cycle does not involve c-Myc function. In embryos, at E13.5, c-Myc-deficient melanocyte precursors are affected in proliferation in concordance with a reduction in numbers, showing that c-Myc is required for the proper melanocyte development. Interestingly, melanocytes from c-Myc-deficient mice display elevated levels of the c-Myc paralog N-Myc. Double deletion of c-Myc and N-Myc results in nearly complete loss of the residual pigmentation, indicating that N-Myc is capable of compensating for c-Myc loss of function in melanocytes.
© 2012 John Wiley & Sons A/S.
Figures

References
-
- Adameyko I, Lallemend F, Aquino JB, et al. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell. 2009;139:366–379. - PubMed
-
- Aydin IT, Beermann F. A MART-1::Cre transgenic line induces recombination in melanocytes and RPE. Genesis. 2011;49:403–409. - PubMed
-
- Bellmeyer A, Krase J, Lindgren J, LaBonne C. The protooncogene c-myc is an essential regulator of neural crest formation in xenopus. Dev Cell. 2003;4:827–839. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

