The spleen is the major source of antidonor antibody-secreting cells in murine heart allograft recipients
- PMID: 22420367
- PMCID: PMC3381891
- DOI: 10.1111/j.1600-6143.2012.04009.x
The spleen is the major source of antidonor antibody-secreting cells in murine heart allograft recipients
Abstract
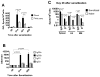
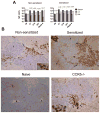
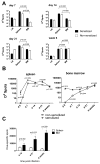
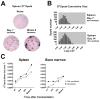
Antibody-mediated allograft rejection is an increasingly recognized problem in clinical transplantation. However, the primary location of donor-specific alloantibody (DSA)-producing cells after transplantation have not been identified. The purpose of this study was to test the contribution of allospecific antibody-secreting cells (ASCs) from different anatomical compartments in a mouse transplantation model. Fully MHC-mismatched heart allografts were transplanted into three groups of recipients: nonsensitized wild type, alloantigen-sensitized wild-type and CCR5(-/-) mice that have exaggerated alloantibody responses. We found that previous sensitization to donor alloantigens resulted in the development of antidonor alloantibody (alloAb) with accelerated kinetics. Nevertheless, the numbers of alloantibody-secreting cells and the serum titers of antidonor IgG alloantibody were equivalent in sensitized and nonsensitized recipients 6 weeks after transplantation. Regardless of recipient sensitization status, the spleen contained higher numbers of donor-reactive ASCs than bone marrow at days 7-21 after transplantation. Furthermore, individual spleen ASCs produced more antidonor IgG alloantibody than bone marrow ASCs. Taken together, our results indicate that the spleen rather than bone marrow is the major source of donor-reactive alloAb early after transplantation in both sensitized and nonsensitized recipients.
© Copyright 2012 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures




References
-
- Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5(10):807–817. - PubMed
-
- Lefaucheur C, Nochy D, Hill GS, Suberbielle-Boissel C, Antoine C, Charron D, et al. Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am J Transplant. 2007;7(4):832–841. - PubMed
-
- Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, et al. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4(7):1033–1041. - PubMed
-
- Lee PC, Terasaki PI, Takemoto SK, Lee PH, Hung CJ, Chen YL, et al. All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation. 2002;74(8):1192–1194. - PubMed
-
- Mauiyyedi S, Pelle PD, Saidman S, Collins AB, Pascual M, Tolkoff-Rubin NE, et al. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001;12(3):574–582. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

