In silico modeling of the pore region of a KCNQ4 missense mutant from a patient with hearing loss
- PMID: 22420747
- PMCID: PMC3374714
- DOI: 10.1186/1756-0500-5-145
In silico modeling of the pore region of a KCNQ4 missense mutant from a patient with hearing loss
Abstract
Background: Mutation of the voltage-gated potassium channel KCNQ4 causes DFNA2-type nonsyndromic autosomal dominant sensorineural hearing loss. KCNQ4 is expressed predominantly in the auditory sensory outer hair cells, which are critical for sound amplification.
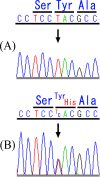
Results: We sequenced KCNQ4 from Japanese patients with sensorineural hearing loss, and identified a novel missense mutation encoding a Tyr270His located at the N-terminus of the highly conserved pore helix sequence. As this patient was not accessible to us and information about them was limited, we used molecular modeling to investigate whether this novel mutation is hypothetically pathogenic. A careful examination of an in silico structural model of the KCNQ4 pore region revealed that the Tyr270His mutation caused an alteration in the electrostatic surface potential of the pore helix.
Conclusion: We propose two possible means by which the Tyr270His mutation causes hearing loss: a positively charged His270 side chain might enhance the helix dipole moment of the pore helix, thereby destabilizing the helix and/or the pore region, or it might disturb transport of K+ through the channel by electrostatic repulsion.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

