Clinical evaluation of gauze-based negative pressure wound therapy in challenging wounds
- PMID: 22420837
- PMCID: PMC7950941
- DOI: 10.1111/j.1742-481X.2012.00955.x
Clinical evaluation of gauze-based negative pressure wound therapy in challenging wounds
Abstract
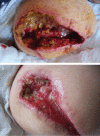
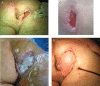
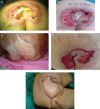
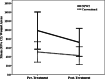
The aim of this randomised clinical study was to evaluate the effectiveness and safety of gauze-based negative pressure wound therapy (NPWT) in patients with challenging wounds. A total of 50 consecutive patients who had wound drainage for more than 5 days, required open wound management and had existence of culture positive infection were included the study. In this study, gauze-based NPWT was compared with conventional dressing therapy in the treatment of patients with difficult-to-heal wounds. The patients were randomly divided into two groups. Group I (n = 25) was followed by conventional antiseptic (polyhexanide solution) dressings, and group II (n = 25) was treated with saline-soaked antibacterial gauze-based NPWT. The wounds' sizes, number of debridement, bacteriology and recurrence were compared between group I and group II. The mean age of the patients was 59·50 years (range 23-97). In group I, average wound sizes of pre- and post-treatment periods were 50·60 ± 55·35 and 42·50 ± 47·92 cm(2), respectively (P < 0·001). Average duration of treatment was 25·52 ± 16·99 days, and average wound size reduction following the treatment was 19·99% in this group. In group II, the wounds displayed considerable shrinkage, accelerated granulation tissue formation, decreased and cleared away exudate. The average wound sizes in the pre- and post-treatment periods were 98·44 ± 100·88 and 72·08 ± 75·78 cm(2) , respectively (P < 0·001). Average duration of treatment was 11·96 ± 2·48 days, and average wound size reduction following the treatment was 32·34%. The patients treated with antibacterial gauze-based NPWT had a significantly reduced recurrence (2 wounds versus 14 wounds, P = 0·001), and increased number of the culture-negative cases (22 wounds versus 16 wounds, P < 0·047) in a follow-up period of 12 months. There was a statistically significant difference between two groups in all measurements. As a result, we can say that the gauze-based NPWT is a safe and effective method in the treatment of challenging infective wounds when compared with conventional wound management.
© 2012 The Authors. International Wound Journal © 2012 Blackwell Publishing Ltd and Medicalhelplines.com Inc.
Figures
References
-
- Krasner DL. Managing wound pain in patients with vacuum‐assisted closure devices. Ostomy Wound Manage 2002;48:38–43. - PubMed
-
- Franczyk M, Lohman RF, Agarwal JP, Rupani G, Drum M, Gottlieb LJ. The impact of topical lidocaine on pain level assessment during and after vacuum‐assisted closure dressing changes; a double‐blind, prospective, randomized study. Plast Reconstr Surg 2009;124:854–61. - PubMed
-
- Tan Y, Wang X, Li H, Zheng Q, Li J, Feng G, Pan Z. The clinical efficacy of the vacuum‐assisted closure therapy in the management of adult osteomyelitis. Arch Orthop Trauma Surg 2011; 131:255–9. - PubMed
-
- Banwell PE. Topical negative pressure therapy in wound care. J Wound Care 1999;8:79–84. - PubMed
-
- Prasarn ML, Zych G, Ostermann PA. Wound management for severe open fractures: use of antibiotic bead pouches and vacuum‐assisted closure. Am J Orthop 2009;38:559–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

