Interim [(18)F]fluorodeoxyglucose positron emission tomography imaging in stage I-II non-bulky Hodgkin lymphoma: would using combined positron emission tomography and computed tomography criteria better predict response than each test alone?
- PMID: 22421007
- PMCID: PMC5751716
- DOI: 10.3109/10428194.2012.676173
Interim [(18)F]fluorodeoxyglucose positron emission tomography imaging in stage I-II non-bulky Hodgkin lymphoma: would using combined positron emission tomography and computed tomography criteria better predict response than each test alone?
Abstract
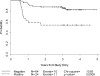
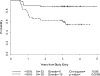
Our objective was to validate the International Harmonization Project (IHP) positron emission tomography (PET) response criteria and correlate with the Deauville criteria and diagnostic computed tomography-based (dCT) lesion size changes. All patients were recruited prospectively to the Cancer and Leukemia Group B (CALGB) 50203 trial for the treatment of stage I-II, non-bulky Hodgkin lymphoma (HL). [(18)F]Fluorodeoxyglucose (FDG) PET and dCT were performed at baseline and after two doxorubicin, vinblastine and gemcitabine (AVG) cycles (PET-2, dCT-2) in 88 patients. IHP and Deauville criteria and percent decrease in the sum of the products of the perpendicular diameters (%SPPD) after two cycles were correlated with progression-free survival (PFS). After a median follow-up of 3.3 years, 23.9% of patients relapsed/progressed (3-year PFS 77%). By IHP, the 2-year PFS was 88% and 54% for PET-2 negative and positive groups, respectively (p = 0.0009). Similar results were obtained for Deauville criteria. In a univariate analysis, PET-2 predicted PFS better than %SPPD, and in a combinatorial analysis, in the PET-2 positive group, a negative dCT-2 increased PFS by 27-35%. However, some confidence intervals were large due to small sample sizes. In conclusion, IHP and Deauville criteria-based interpretation of PET-2 was strongly associated with 2-year PFS. The combined analysis of PET-2 with dCT-2 suggested a better predictive value for PFS compared to either test alone. Further studies are under way to confirm these findings.
Figures


Comment in
-
Interim response assessment for Hodgkin lymphoma: size matters.Leuk Lymphoma. 2012 Nov;53(11):2095-6. doi: 10.3109/10428194.2012.701296. Epub 2012 Jul 9. Leuk Lymphoma. 2012. PMID: 22680772 No abstract available.
References
-
- Armitage JO, Weisenburger DD, Hutchins M, et al. Chemotherapy for diffuse large-cell lymphoma–rapidly responding patients have more durable remissions. J ClinOncol. 1986;4:160–164. - PubMed
-
- Haw R, Sawka CA, Franssen E, Berinstein HL. Significance of a partial or slow response to front-line chemotherapy in the management of intermediate-grade or high-grade non-Hodgkin’s lymphoma: a literature review. J ClinOncol. 1994;12:1074–1084. - PubMed
-
- Meyer RM, Gospodarowicz MK, Connors JM, et al. Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage Hodgkin’s lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. J ClinOncol. 2005;23:4634–4642. - PubMed
-
- Radford JA, Cowan RA, Flanagan M, et al. The significance of residual mediastinal abnormality on the chest radiograph following treatment for Hodgkin’s disease. J ClinOncol. 1988;6:940–946. - PubMed
-
- Lewis E, Bernardino ME, Salvador PG, Cabanillas FF, Barnes PA, Thomas JL. Post-therapy CTdetected mass in lymphoma patients: is it viable tissue? J Comput Assist Tomogr. 1982;6:792–795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA032291/CA/NCI NIH HHS/United States
- U10 CA059518/CA/NCI NIH HHS/United States
- U10 CA045418/CA/NCI NIH HHS/United States
- U10 CA077440/CA/NCI NIH HHS/United States
- CA77440/CA/NCI NIH HHS/United States
- U10 CA041287/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- CA77658/CA/NCI NIH HHS/United States
- U10 CA047642/CA/NCI NIH HHS/United States
- CA33601/CA/NCI NIH HHS/United States
- CA77597/CA/NCI NIH HHS/United States
- CA04457/CA/NCI NIH HHS/United States
- U10 CA077658/CA/NCI NIH HHS/United States
- CA32291/CA/NCI NIH HHS/United States
- U10 CA086726/CA/NCI NIH HHS/United States
- U10 CA045808/CA/NCI NIH HHS/United States
- U10 CA007968/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- CA77651/CA/NCI NIH HHS/United States
- U10 CA045389/CA/NCI NIH HHS/United States
- U10 CA021060/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA077597/CA/NCI NIH HHS/United States
- U10 CA047559/CA/NCI NIH HHS/United States
- U10 CA077651/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- U10 CA003927/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
