Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor
- PMID: 22421133
- PMCID: PMC3323948
- DOI: 10.1073/pnas.1118959109
Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor
Abstract
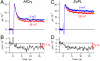
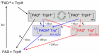
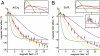
Among the biological phenomena that fall within the emerging field of "quantum biology" is the suggestion that magnetically sensitive chemical reactions are responsible for the magnetic compass of migratory birds. It has been proposed that transient radical pairs are formed by photo-induced electron transfer reactions in cryptochrome proteins and that their coherent spin dynamics are influenced by the geomagnetic field leading to changes in the quantum yield of the signaling state of the protein. Despite a variety of supporting evidence, it is still not clear whether cryptochromes have the properties required to respond to magnetic interactions orders of magnitude weaker than the thermal energy, k(B)T. Here we demonstrate that the kinetics and quantum yields of photo-induced flavin-tryptophan radical pairs in cryptochrome are indeed magnetically sensitive. The mechanistic origin of the magnetic field effect is clarified, its dependence on the strength of the magnetic field measured, and the rates of relevant spin-dependent, spin-independent, and spin-decoherence processes determined. We argue that cryptochrome is fit for purpose as a chemical magnetoreceptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ahmad M, Cashmore AR. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. - PubMed
-
- Chaves I, et al. The cryptochromes: Blue light photoreceptors in plants and animals. Annu Rev Plant Biol. 2011;62:335–364. - PubMed
-
- Weber S. Light-driven enzymatic catalysis of DNA repair: A review of recent biophysical studies on photolyase. Biochim Biophys Acta. 2005;1707:1–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

