Cannabinoid receptors: nomenclature and pharmacological principles
- PMID: 22421596
- PMCID: PMC3378782
- DOI: 10.1016/j.pnpbp.2012.02.009
Cannabinoid receptors: nomenclature and pharmacological principles
Abstract
The CB1 and CB2 cannabinoid receptors are members of the G protein-coupled receptor (GPCR) family that are pharmacologically well defined. However, the discovery of additional sites of action for endocannabinoids as well as synthetic cannabinoid compounds suggests the existence of additional cannabinoid receptors. Here we review this evidence, as well as the current nomenclature for classifying a target as a cannabinoid receptor. Basic pharmacological definitions, principles and experimental conditions are discussed in order to place in context the mechanisms underlying cannabinoid receptor activation. Constitutive (agonist-independent) activity is observed with the overexpression of many GPCRs, including cannabinoid receptors. Allosteric modulators can alter the pharmacological responses of cannabinoid receptors. The complex molecular architecture of each of the cannabinoid receptors allows for a single receptor to recognize multiple classes of compounds and produce an array of distinct downstream effects. Natural polymorphisms and alternative splice variants may also contribute to their pharmacological diversity. As our knowledge of the distinct differences grows, we may be able to target select receptor conformations and their corresponding pharmacological responses. Importantly, the basic biology of the endocannabinoid system will continue to be revealed by ongoing investigations.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



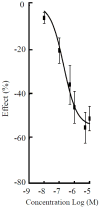
References
-
- Abood ME. Molecular biology of cannabinoid receptors. Handbook of experimental pharmacology. 2005:81–115. - PubMed
-
- Abood ME, Ditto KA, Noel MA, Showalter VM, Tao Q. Isolation and expression of mouse CB1 cannabinoid receptor gene: comparison of binding properties with those of native CB1 receptors in mouse brain and N18TG2 neuroblastoma cells. Biochem Pharmacol. 1997;53:207–214. - PubMed
-
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. - PMC - PubMed
-
- Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci. 2006;27:1–4. Epub 2005 Nov 2028. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

