The zinc finger protein ZPR1 is a potential modifier of spinal muscular atrophy
- PMID: 22422766
- PMCID: PMC3363332
- DOI: 10.1093/hmg/dds102
The zinc finger protein ZPR1 is a potential modifier of spinal muscular atrophy
Abstract
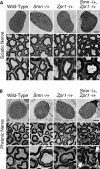
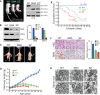
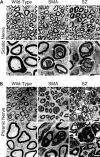
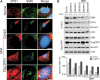
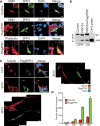
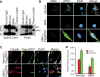
Spinal muscular atrophy (SMA) is caused by mutation of the Survival Motor Neurons 1 (SMN1) gene and is characterized by degeneration of spinal motor neurons. The severity of SMA is primarily influenced by the copy number of the SMN2 gene. Additional modifier genes that lie outside the SMA locus exist and one gene that could modify SMA is the Zinc Finger Protein (ZPR1) gene. To test the significance of ZPR1 downregulation in SMA, we examined the effect of reduced ZPR1 expression in mice with mild and severe SMA. We report that the reduced ZPR1 expression causes increase in the loss of motor neurons, hypermyelination in phrenic nerves, increase in respiratory distress and disease severity and reduces the lifespan of SMA mice. The deficiency of SMN-containing sub-nuclear bodies correlates with the severity of SMA. ZPR1 is required for the accumulation of SMN in sub-nuclear bodies. Further, we report that ZPR1 overexpression increases levels of SMN and promotes accumulation of SMN in sub-nuclear bodies in SMA patient fibroblasts. ZPR1 stimulates neurite growth and rescues axonal growth defects in SMN-deficient spinal cord neurons from SMA mice. These data suggest that the severity of disease correlates negatively with ZPR1 levels and ZPR1 may be a protective modifier of SMA.
Figures

References
-
- Munsat T.L., Davies K.E. International SMA consortium meeting (26–28 June 1992, Bonn, Germany) Neuromuscul. Disord. 1992;2:423–428. - PubMed
-
- Lefebvre S., Burglen L., Reboullet S., Clermont O., Burlet P., Viollet L., Benichou B., Cruaud C., Millasseau P., Zeviani M., et al. Identification and characterization of a spinal muscular atrophy- determining gene. Cell. 1995;80:155–165. - PubMed
-
- Lefebvre S., Burlet P., Liu Q., Bertrandy S., Clermont O., Munnich A., Dreyfuss G., Melki J. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat. Genet. 1997;16:265–269. - PubMed
-
- Monani U.R., Lorson C.L., Parsons D.W., Prior T.W., Androphy E.J., Burghes A.H., McPherson J.D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999;8:1177–1183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

