Comparison of seven tests for high-grade cervical intraepithelial neoplasia in women with abnormal smears: the Predictors 2 study
- PMID: 22422852
- PMCID: PMC3372127
- DOI: 10.1128/JCM.00181-12
Comparison of seven tests for high-grade cervical intraepithelial neoplasia in women with abnormal smears: the Predictors 2 study
Abstract
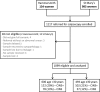
High-risk human papillomavirus (HPV) DNA/RNA testing provides higher sensitivity but lower specificity than cytology for the identification of high-grade cervical intraepithelial neoplasia (CIN). Several new HPV tests are now available for this purpose, and a direct comparison of their properties is needed. Seven tests were evaluated with samples in liquid PreservCyt transport medium from 1,099 women referred for colposcopy: the Hybrid Capture 2 (Qiagen), Cobas (Roche), PreTect HPV-Proofer (NorChip), Aptima HPV (Gen-Probe), and Abbott RealTime assays, the BD HPV test, and CINtec p16(INK4a) cytology (mtm laboratories) immunocytochemistry. Sensitivity, specificity, and positive predictive value (PPV) were based on the worst histology found on either the biopsy or the treatment specimen after central review. Three hundred fifty-nine women (32.7%) had CIN grade 2+ (CIN2+), with 224 (20.4%) having CIN3+. For detection of CIN2+, Hybrid Capture 2 had 96.3% sensitivity, 19.5% specificity, and 37.4% PPV. Cobas had 95.2% sensitivity, 24.0% specificity, and 37.6% PPV. The BD HPV test had 95.0% sensitivity, 24.2% specificity, and 37.8% PPV. Abbott RealTime had 93.3% sensitivity, 27.3% specificity, and 38.2% PPV. Aptima had 95.3% sensitivity, 28.8% specificity, and 39.3% PPV. PreTect HPV-Proofer had 74.1% sensitivity, 70.8% specificity, and 55.4% PPV. CINtec p16(INK4a) cytology had 85.7% sensitivity, 54.7% specificity, and 49.1% PPV. Cytology of a specimen taken at colposcopy (mild dyskaryosis or worse) had 88.9% sensitivity, 58.1% specificity, and 50.7% PPV. Our study confirms that, in a referral setting, HPV testing by a number of different tests provides high sensitivity for high-grade disease. Further work is needed to confirm these findings in a routine screening setting.
Figures

References
-
- Almonte M, et al. 2007. Cervical screening by visual inspection, HPV testing, liquid-based and conventional cytology in Amazonian Peru. Int. J. Cancer 121:796–802 - PubMed
-
- Bulkmans NW, et al. 2007. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 370:1764–1772 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

