Pleiotropic IFN-dependent and -independent effects of IRF5 on the pathogenesis of experimental lupus
- PMID: 22422888
- PMCID: PMC3580234
- DOI: 10.4049/jimmunol.1103113
Pleiotropic IFN-dependent and -independent effects of IRF5 on the pathogenesis of experimental lupus
Abstract
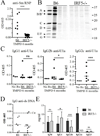
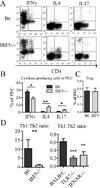
Genetic polymorphisms of IFN regulatory factor 5 (IRF5) are associated with an increased risk of lupus in humans. In this study, we examined the role of IRF5 in the pathogenesis of pristane-induced lupus in mice. The pathological response to pristane in IRF5(-/-) mice shared many features with type I IFN receptor (IFNAR)(-/-) and TLR7(-/-) mice: production of anti-Sm/RNP autoantibodies, glomerulonephritis, generation of Ly6C(hi) monocytes, and IFN-I production all were greatly attenuated. Lymphocyte activation following pristane injection was greatly diminished in IRF5(-/-) mice, and Th cell differentiation was deviated from Th1 in wild-type mice toward Th2 in IRF5(-/-) mice. Th cell development was skewed similarly in TLR7(-/-) or IFNAR(-/-) mice, suggesting that IRF5 alters T cell activation and differentiation by affecting cytokine production. Indeed, production of IFN-I, IL-12, and IL-23 in response to pristane was markedly decreased, whereas IL-4 increased. Unexpectedly, plasmacytoid dendritic cells (pDC) were not recruited to the site of inflammation in IRF5(-/-) or MyD88(-/-) mice, but were recruited normally in IFNAR(-/-) and TLR7(-/-) mice. In striking contrast to wild-type mice, pristane did not stimulate local expression of CCL19 and CCL21 in IRF5(-/-) mice, suggesting that IRF5 regulates chemokine-mediated pDC migration independently of its effects on IFN-I. Collectively, these data indicate that altered production of IFN-I and other cytokines in IRF5(-/-) mice prevents pristane from inducing lupus pathology by broadly affecting T and B lymphocyte activation/differentiation. Additionally, we uncovered a new, IFN-I-independent role of IRF5 in regulating chemokines involved in the homing of pDCs and certain lymphocyte subsets.
Figures





References
-
- Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S, Honda K, Ohba Y, Mak TW, Taniguchi T. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. - PubMed
-
- Barnes BJ, Moore PA, Pitha PM. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J Biol Chem. 2001;276:23382–23390. - PubMed
-
- Schoenemeyer A, Barnes BJ, Mancl ME, Latz E, Goutagny N, Pitha PM, Fitzgerald KA, Golenbock DT. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J Biol Chem. 2005;280:17005–17012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

