A model for personalized in vivo analysis of human immune responsiveness
- PMID: 22422991
- PMCID: PMC3697150
- DOI: 10.1126/scitranslmed.3003481
A model for personalized in vivo analysis of human immune responsiveness
Abstract
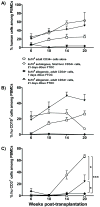
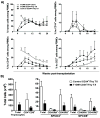
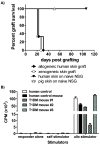
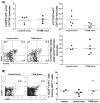
Studies of human immune diseases are generally limited to the analysis of peripheral blood lymphocytes of heterogeneous patient populations. Improved models are needed to allow analysis of fundamental immunologic abnormalities predisposing to disease and in which to assess immunotherapies. Immunodeficient mice receiving human fetal thymus grafts and fetal CD34(+) cells intravenously produce robust human immune systems, allowing analysis of human T cell development and function. However, to use humanized mice to study human immune-mediated disorders, immune systems must be generated from adult hematopoietic cells. Here, we demonstrated robust immune reconstitution in mice with hematopoietic stem cells (HSCs) aspirated from bone marrow of adults with type 1 diabetes (T1D) and healthy control volunteers. In these humanized mice, cryopreservation of human leukocyte antigen allele-matched fetal thymic tissue prevented allogeneic adult HSC rejection. Newly generated T cells, which included regulatory T cells (T(regs)), were functional and self-tolerant and had a diverse repertoire. The immune recognition of these mice mimicked that of the adult CD34(+) cell donor, but the T cell phenotypes were more predominantly "naïve" than those of the adult donors. HSCs from T1D and control donors generated similar numbers of natural T(regs) intrathymically; however, peripheral T cells from T1D subjects showed increased proportions of activated or memory cells compared to controls, suggesting possible HSC-intrinsic differences in T cell homeostasis that might underlie immune pathology in T1D. This "personalized immune" mouse provides a new model for individualized analysis of human immune responses that may provide new insights into not only T1D but also other forms of immune function and dysfunction as well.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. - PubMed
-
- McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: Murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. - PubMed
-
- Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–492. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

