Data-driven prediction of drug effects and interactions
- PMID: 22422992
- PMCID: PMC3382018
- DOI: 10.1126/scitranslmed.3003377
Data-driven prediction of drug effects and interactions
Abstract
Adverse drug events remain a leading cause of morbidity and mortality around the world. Many adverse events are not detected during clinical trials before a drug receives approval for use in the clinic. Fortunately, as part of postmarketing surveillance, regulatory agencies and other institutions maintain large collections of adverse event reports, and these databases present an opportunity to study drug effects from patient population data. However, confounding factors such as concomitant medications, patient demographics, patient medical histories, and reasons for prescribing a drug often are uncharacterized in spontaneous reporting systems, and these omissions can limit the use of quantitative signal detection methods used in the analysis of such data. Here, we present an adaptive data-driven approach for correcting these factors in cases for which the covariates are unknown or unmeasured and combine this approach with existing methods to improve analyses of drug effects using three test data sets. We also present a comprehensive database of drug effects (Offsides) and a database of drug-drug interaction side effects (Twosides). To demonstrate the biological use of these new resources, we used them to identify drug targets, predict drug indications, and discover drug class interactions. We then corroborated 47 (P < 0.0001) of the drug class interactions using an independent analysis of electronic medical records. Our analysis suggests that combined treatment with selective serotonin reuptake inhibitors and thiazides is associated with significantly increased incidence of prolonged QT intervals. We conclude that confounding effects from covariates in observational clinical data can be controlled in data analyses and thus improve the detection and prediction of adverse drug effects and interactions.
Figures


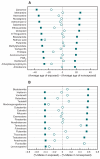
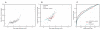
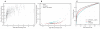
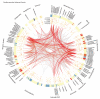

References
-
- Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, Laffel G, Sweitzer BJ, Shea BF, Hallisey R, Vliet MV, Nemeskal R, Leape LL, ADE Prevention Study Group. Leape LL, Servi D, Laird N, Bates D, Hojnowski-Diaz P, Petersen LA, Petrycki S, Vliet MV, Cotugno M, Patterson H, Shea BF, Hickey M, Kleefield S, Cooper J, Cullen DJ, Kinneally E, Nemeskal R, Sweitzer BJ, Small SD, Demonaco HJ, Clapp MD, Hallisey R, Gallivan T, Ives J, Porter K, Thompson BT, Laffel G, Hackman JR, Edmondson A. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. JAMA. 1995;274:29–34. - PubMed
-
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277:301–306. - PubMed
-
- Szarfman A, Tonning JM, Doraiswamy PM. Pharmacovigilance in the 21st century: New systematic tools for an old problem. Pharmacotherapy. 2004;24:1099–1104. - PubMed
-
- Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol. Drug Saf. 2009;18:427–436. - PubMed
-
- Szarfman A, Machado SG, O’Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf. 2002;25:381–392. - PubMed
Publication types
MeSH terms
Grants and funding
- R24 GM061374/GM/NIGMS NIH HHS/United States
- RR025742/RR/NCRR NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- LM05652/LM/NLM NIH HHS/United States
- T15 LM007033/LM/NLM NIH HHS/United States
- UL1 RR025744/RR/NCRR NIH HHS/United States
- UL1 TR001085/TR/NCATS NIH HHS/United States
- R24GM61374/GM/NIGMS NIH HHS/United States
- TL1 TR001084/TR/NCATS NIH HHS/United States
- TL1 RR025742/RR/NCRR NIH HHS/United States
- LM007033/LM/NLM NIH HHS/United States
- R01 LM005652/LM/NLM NIH HHS/United States
- P50 MH094267/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

