NMDAR antagonist action in thalamus imposes δ oscillations on the hippocampus
- PMID: 22423006
- PMCID: PMC3378362
- DOI: 10.1152/jn.00072.2012
NMDAR antagonist action in thalamus imposes δ oscillations on the hippocampus
Abstract
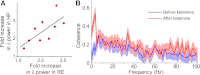
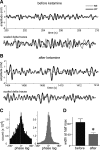
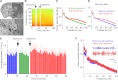
Work on schizophrenia demonstrates the involvement of the hippocampus in the disease and points specifically to hyperactivity of CA1. Many symptoms of schizophrenia can be mimicked by N-methyl-d-aspartate receptor (NMDAR) antagonist; notably, delta frequency oscillations in the awake state are enhanced in schizophrenia, an abnormality that can be mimicked by NMDAR antagonist action in the thalamus. Given that CA1 receives input from the nucleus reuniens of the thalamus, we sought to determine whether an NMDAR antagonist in the thalamus can affect hippocampal processes. We found that a systemic NMDAR antagonist (ketamine; 50 mg/kg) increased the firing rate of cells in the reuniens and CA1 in awake rats. Furthermore, ketamine increased the power of delta oscillations in both structures. The thalamic origin of the change in hippocampal properties was demonstrated in three ways: 1) oscillations in the two structures were coherent; 2) the hippocampal changes induced by systematic ketamine were reduced by thalamic injection of muscimol; and 3) the hippocampal changes could be induced by local injection of ketamine into the thalamus. Lower doses of ketamine (20 mg/kg) did not evoke delta oscillations but did increase hippocampal gamma power, an effect not dependent on the thalamus. There are thus at least two mechanisms for ketamine action on the hippocampus: a low-dose mechanism that affects gamma through a nonthalamic mechanism and a high-dose mechanism that increases CA1 activity and delta oscillations as a result of input from the thalamus. Both mechanisms may be important in producing symptoms of schizophrenia.
Figures



Similar articles
-
Delta frequency optogenetic stimulation of the thalamic nucleus reuniens is sufficient to produce working memory deficits: relevance to schizophrenia.Biol Psychiatry. 2015 Jun 15;77(12):1098-107. doi: 10.1016/j.biopsych.2015.01.020. Epub 2015 Feb 28. Biol Psychiatry. 2015. PMID: 25891221 Free PMC article. Review.
-
Neuronal correlates of ketamine and walking induced gamma oscillations in the medial prefrontal cortex and mediodorsal thalamus.PLoS One. 2017 Nov 2;12(11):e0186732. doi: 10.1371/journal.pone.0186732. eCollection 2017. PLoS One. 2017. PMID: 29095852 Free PMC article.
-
Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia.Brain Struct Funct. 2012 Apr;217(2):395-409. doi: 10.1007/s00429-011-0351-8. Epub 2011 Oct 7. Brain Struct Funct. 2012. PMID: 21979451 Free PMC article.
-
A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia.Biol Psychiatry. 2010 Jul 1;68(1):17-24. doi: 10.1016/j.biopsych.2010.04.007. Epub 2010 May 31. Biol Psychiatry. 2010. PMID: 20553749 Free PMC article. Review.
-
Potential synergistic action of 19 schizophrenia risk genes in the thalamus.Schizophr Res. 2017 Feb;180:64-69. doi: 10.1016/j.schres.2016.09.008. Epub 2016 Sep 16. Schizophr Res. 2017. PMID: 27645107 Free PMC article. Review.
Cited by
-
Machine learning of EEG spectra classifies unconsciousness during GABAergic anesthesia.PLoS One. 2021 May 6;16(5):e0246165. doi: 10.1371/journal.pone.0246165. eCollection 2021. PLoS One. 2021. PMID: 33956800 Free PMC article.
-
Electroencephalogram signatures of ketamine anesthesia-induced unconsciousness.Clin Neurophysiol. 2016 Jun;127(6):2414-22. doi: 10.1016/j.clinph.2016.03.005. Epub 2016 Mar 16. Clin Neurophysiol. 2016. PMID: 27178861 Free PMC article.
-
Delta frequency optogenetic stimulation of the thalamic nucleus reuniens is sufficient to produce working memory deficits: relevance to schizophrenia.Biol Psychiatry. 2015 Jun 15;77(12):1098-107. doi: 10.1016/j.biopsych.2015.01.020. Epub 2015 Feb 28. Biol Psychiatry. 2015. PMID: 25891221 Free PMC article. Review.
-
Recent insights into the mode of action of memantine and ketamine.Curr Opin Pharmacol. 2015 Feb;20:54-63. doi: 10.1016/j.coph.2014.11.006. Epub 2014 Dec 2. Curr Opin Pharmacol. 2015. PMID: 25462293 Free PMC article. Review.
-
Ketamine Dysregulates the Amplitude and Connectivity of High-Frequency Oscillations in Cortical-Subcortical Networks in Humans: Evidence From Resting-State Magnetoencephalography-Recordings.Schizophr Bull. 2015 Sep;41(5):1105-14. doi: 10.1093/schbul/sbv051. Epub 2015 May 18. Schizophr Bull. 2015. PMID: 25987642 Free PMC article. Clinical Trial.
References
-
- Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci 9: 902–911, 1997 - PubMed
-
- Buzsáki G. The thalamic clock: emergent network properties. Neuroscience 41: 351–364, 1991 - PubMed
-
- Clementz BA, Sponheim SR, Iacono WG, Beiser M. Resting EEG in first-episode schizophrenia patients, bipolar psychosis patients, and their first-degree relatives. Psychophysiology 31: 486–494, 1994 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

