Motor neuron rescue in spinal muscular atrophy mice demonstrates that sensory-motor defects are a consequence, not a cause, of motor neuron dysfunction
- PMID: 22423102
- PMCID: PMC3679185
- DOI: 10.1523/JNEUROSCI.5775-11.2012
Motor neuron rescue in spinal muscular atrophy mice demonstrates that sensory-motor defects are a consequence, not a cause, of motor neuron dysfunction
Abstract
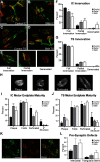
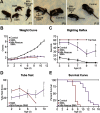
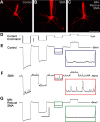
The loss of motor neurons (MNs) is a hallmark of the neuromuscular disease spinal muscular atrophy (SMA); however, it is unclear whether this phenotype autonomously originates within the MN. To address this question, we developed an inducible mouse model of severe SMA that has perinatal lethality, decreased motor function, motor unit pathology, and hyperexcitable MNs. Using an Hb9-Cre allele, we increased Smn levels autonomously within MNs and demonstrate that MN rescue significantly improves all phenotypes and pathologies commonly described in SMA mice. MN rescue also corrects hyperexcitability in SMA motor neurons and prevents sensory-motor synaptic stripping. Survival in MN-rescued SMA mice is extended by only 5 d, due in part to failed autonomic innervation of the heart. Collectively, this work demonstrates that the SMA phenotype autonomously originates in MNs and that sensory-motor synapse loss is a consequence, not a cause, of MN dysfunction.
Figures




Similar articles
-
Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy.Neuron. 2011 Feb 10;69(3):453-67. doi: 10.1016/j.neuron.2010.12.032. Neuron. 2011. PMID: 21315257 Free PMC article.
-
Reduced survival of motor neuron (SMN) protein in motor neuronal progenitors functions cell autonomously to cause spinal muscular atrophy in model mice expressing the human centromeric (SMN2) gene.J Neurosci. 2010 Sep 8;30(36):12005-19. doi: 10.1523/JNEUROSCI.2208-10.2010. J Neurosci. 2010. PMID: 20826664 Free PMC article.
-
Improvement of neuromuscular synaptic phenotypes without enhanced survival and motor function in severe spinal muscular atrophy mice selectively rescued in motor neurons.PLoS One. 2013 Sep 23;8(9):e75866. doi: 10.1371/journal.pone.0075866. eCollection 2013. PLoS One. 2013. PMID: 24086650 Free PMC article.
-
At the "junction" of spinal muscular atrophy pathogenesis: the role of neuromuscular junction dysfunction in SMA disease progression.Curr Mol Med. 2013 Aug;13(7):1160-74. doi: 10.2174/15665240113139990044. Curr Mol Med. 2013. PMID: 23514457 Review.
-
Beyond Motor Neurons in Spinal Muscular Atrophy: A Focus on Neuromuscular Junction.Int J Mol Sci. 2024 Jul 3;25(13):7311. doi: 10.3390/ijms25137311. Int J Mol Sci. 2024. PMID: 39000416 Free PMC article. Review.
Cited by
-
Spinal muscular atrophy patient-derived motor neurons exhibit hyperexcitability.Sci Rep. 2015 Jul 20;5:12189. doi: 10.1038/srep12189. Sci Rep. 2015. PMID: 26190808 Free PMC article.
-
Molecular Mechanisms of Neurodegeneration in Spinal Muscular Atrophy.J Exp Neurosci. 2016 Mar 23;10:39-49. doi: 10.4137/JEN.S33122. eCollection 2016. J Exp Neurosci. 2016. PMID: 27042141 Free PMC article. Review.
-
Motor neuron cell-nonautonomous rescue of spinal muscular atrophy phenotypes in mild and severe transgenic mouse models.Genes Dev. 2015 Feb 1;29(3):288-97. doi: 10.1101/gad.256644.114. Epub 2015 Jan 12. Genes Dev. 2015. PMID: 25583329 Free PMC article.
-
Suppression of the necroptotic cell death pathways improves survival in Smn 2B/- mice.Front Cell Neurosci. 2022 Aug 3;16:972029. doi: 10.3389/fncel.2022.972029. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35990890 Free PMC article.
-
Upper motor neurons are a target for gene therapy and UCHL1 is necessary and sufficient to improve cellular integrity of diseased upper motor neurons.Gene Ther. 2022 Apr;29(3-4):178-192. doi: 10.1038/s41434-021-00303-4. Epub 2021 Dec 2. Gene Ther. 2022. PMID: 34853443 Free PMC article.
References
-
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
