Prolonged disynaptic inhibition in the cortex mediated by slow, non-α7 nicotinic excitation of a specific subset of cortical interneurons
- PMID: 22423106
- PMCID: PMC3320796
- DOI: 10.1523/JNEUROSCI.0115-12.2012
Prolonged disynaptic inhibition in the cortex mediated by slow, non-α7 nicotinic excitation of a specific subset of cortical interneurons
Abstract
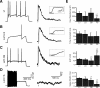
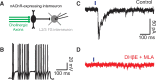
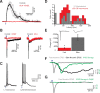
Cholinergic activation of nicotinic receptors in the cortex plays a critical role in arousal, attention, and learning. Here we demonstrate that cholinergic axons from the basal forebrain of mice excite a specific subset of cortical interneurons via a remarkably slow, non-α7 nicotinic receptor-mediated conductance. In turn, these inhibitory cells generate a delayed and prolonged wave of disynaptic inhibition in neighboring cortical neurons, altering the spatiotemporal pattern of inhibition in cortical circuits.
Figures

References
-
- Alkondon M, Pereira EF, Albuquerque EX. alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. - PubMed
-
- Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9:178–183. - PubMed
-
- Blaizot X, Landeau B, Baron JC, Chavoix C. Mapping the visual recognition memory network with PET in the behaving baboon. J Cereb Blood Flow Metab. 2000;20:213–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
