Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine
- PMID: 22423139
- PMCID: PMC3404712
- DOI: 10.1093/cid/cis307
Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine
Abstract
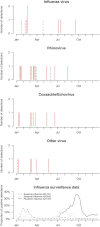
We randomized 115 children to trivalent inactivated influenza vaccine (TIV) or placebo. Over the following 9 months, TIV recipients had an increased risk of virologically-confirmed non-influenza infections (relative risk: 4.40; 95% confidence interval: 1.31-14.8). Being protected against influenza, TIV recipients may lack temporary non-specific immunity that protected against other respiratory viruses.
Figures
References
-
- Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2008;(2):CD004879. - PubMed
-
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. - PubMed
-
- Glezen P, Denny FW. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288:498–505. - PubMed
-
- Glezen WP, Paredes A, Taber LH. Influenza in children. Relationship to other respiratory agents. JAMA. 1980;243:1345–9. - PubMed
-
- Anestad G. Interference between outbreaks of respiratory syncytial virus and influenza virus infection. Lancet. 1982;1:502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

