Modelling the dynamics of Plasmodium falciparum histidine-rich protein 2 in human malaria to better understand malaria rapid diagnostic test performance
- PMID: 22423618
- PMCID: PMC3359291
- DOI: 10.1186/1475-2875-11-74
Modelling the dynamics of Plasmodium falciparum histidine-rich protein 2 in human malaria to better understand malaria rapid diagnostic test performance
Abstract
Background: Effective diagnosis of malaria is a major component of case management. Rapid diagnostic tests (RDTs) based on Plasmodium falciparumhistidine-rich protein 2 (PfHRP2) are popular for diagnosis of this most virulent malaria infection. However, concerns have been raised about the longevity of the PfHRP2 antigenaemia following curative treatment in endemic regions.
Methods: A model of PfHRP2 production and decay was developed to mimic the kinetics of PfHRP2 antigenaemia during infections. Data from two human infection studies was used to fit the model, and to investigate PfHRP2 kinetics. Four malaria RDTs were assessed in the laboratory to determine the minimum detectable concentration of PfHRP2.
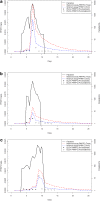
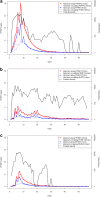
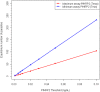
Results: Fitting of the PfHRP2 dynamics model indicated that in malaria naïve hosts, P. falciparum parasites of the 3D7 strain produce 1.4 × 10⁻¹³ g of PfHRP2 per parasite per replication cycle. The four RDTs had minimum detection thresholds between 6.9 and 27.8 ng/mL. Combining these detection thresholds with the kinetics of PfHRP2, it is predicted that as few as 8 parasites/μL may be required to maintain a positive RDT in a chronic infection.
Conclusions: The results of the model indicate that good quality PfHRP2-based RDTs should be able to detect parasites on the first day of symptoms, and that the persistence of the antigen will cause the tests to remain positive for at least seven days after treatment. The duration of a positive test result following curative treatment is dependent on the duration and density of parasitaemia prior to treatment and the presence and affinity of anti-PfHRP2 antibodies.
Figures
References
-
- World Health Organization. Guidelines for the treatment of malaria. Geneva. 2 2010. - PubMed
-
- Abeku TA, Kristan M, Jones C, Beard J, Mueller DH, Okia M, Rapuoda B, Greenwood B, Cox J. Determinants of the accuracy of rapid diagnostic tests in malaria case management: evidence from low and moderate transmission settings in the East African highlands. Malar J. 2008;7:202. doi: 10.1186/1475-2875-7-202. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

