Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson's disease
- PMID: 22424171
- PMCID: PMC4530392
- DOI: 10.1089/hum.2011.220
Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson's disease
Abstract
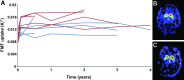
We report the results of a long-term follow-up of subjects in a phase 1 study of AAV2-hAADC (adeno-associated virus type 2-human aromatic L-amino acid decarboxylase) gene therapy for the treatment of Parkinson's disease (PD). Ten patients with moderately advanced PD received bilateral putaminal infusions of either a low or a high dose of AAV2-hAADC vector. An annual positron emission tomography (PET) imaging with [(18)F]fluoro-L-m-tyrosine tracer was used for evaluation of AADC expression, and a standard clinical rating scale [Unified Parkinson's Disease Rating Scale (UPDRS)] was used to assess effect. Our previous analysis of the 6-month data suggested that this treatment was acutely safe and well tolerated. We found that the elevated PET signal observed in the first 12 months persisted over 4 years in both dose groups. A significantly increased PET value compared with the presurgery baseline was maintained over the 4-year monitoring period. The UPDRS in all patients off medication for 12 hr improved in the first 12 months, but displayed a slow deterioration in subsequent years. This analysis demonstrates that apparent efficacy continues through later years with an acceptable safety profile. These data indicate stable transgene expression over 4 years after vector delivery and continued safety, but emphasize the need for a controlled efficacy trial and the use of a higher vector dose.
Figures

Comment in
-
Challenges for gene therapy of CNS disorders and implications for Parkinson's disease therapies.Hum Gene Ther. 2012 Apr;23(4):340-3. doi: 10.1089/hum.2012.2507. Epub 2012 Apr 10. Hum Gene Ther. 2012. PMID: 22490128 No abstract available.
References
-
- Bankiewicz K.S. Eberling J.L. Kohutnicka M., et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp. Neurol. 2000;164:2–14. - PubMed
-
- Bankiewicz K.S. Forsayeth J. Eberling J.L., et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol. Ther. 2006;14:564–570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

