Cellular metabolism and disease: what do metabolic outliers teach us?
- PMID: 22424225
- PMCID: PMC3337773
- DOI: 10.1016/j.cell.2012.02.032
Cellular metabolism and disease: what do metabolic outliers teach us?
Abstract
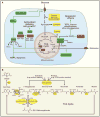
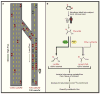
An understanding of metabolic pathways based solely on biochemistry textbooks would underestimate the pervasive role of metabolism in essentially every aspect of biology. It is evident from recent work that many human diseases involve abnormal metabolic states--often genetically programmed--that perturb normal physiology and lead to severe tissue dysfunction. Understanding these metabolic outliers is now a crucial frontier in disease-oriented research. This Review discusses the broad impact of metabolism in cellular function and how modern concepts of metabolism can inform our understanding of common diseases like cancer and also considers the prospects of developing new metabolic approaches to disease treatment.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Adam J, Hatipoglu E, O’Flaherty L, Ternette N, Sahgal N, Lockstone H, Baban D, Nye E, Stamp GW, Wolhuter K, et al. Renal Cyst Formation in Fh1-Deficient Mice Is Independent of the Hif/Phd Pathway: Roles for Fumarate in KEAP1 Succination and Nrf2 Signaling. Cancer Cell. 2011;20:524–537. - PMC - PubMed
-
- Alderson NL, Wang Y, Blatnik M, Frizzell N, Walla MD, Lyons TJ, Alt N, Carson JA, Nagai R, Thorpe SR, et al. S-(2-Succinyl)cysteine: a novel chemical modification of tissue proteins by a Krebs cycle intermediate. Arch Biochem Biophys. 2006;450:1–8. - PubMed
-
- Alfardan J, Mohsen AW, Copeland S, Ellison J, Keppen-Davis L, Rohrbach M, Powell BR, Gillis J, Matern D, Kant J, et al. Characterization of new ACADSB gene sequence mutations and clinical implications in patients with 2-methylbutyrylglycinuria identified by newborn screening. Mol Genet Metab. 2010;100:333–338. - PMC - PubMed
-
- Baysal BE. Clinical and molecular progress in hereditary paraganglioma. J Med Genet. 2008;45:689–694. - PubMed
-
- Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

