Outwitting evolution: fighting drug-resistant TB, malaria, and HIV
- PMID: 22424234
- PMCID: PMC3322542
- DOI: 10.1016/j.cell.2012.02.021
Outwitting evolution: fighting drug-resistant TB, malaria, and HIV
Abstract
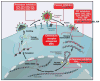
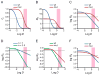
Although caused by vastly different pathogens, the world's three most serious infectious diseases, tuberculosis, malaria, and HIV-1 infection, share the common problem of drug resistance. The pace of drug development has been very slow for tuberculosis and malaria and rapid for HIV-1. But for each disease, resistance to most drugs has appeared quickly after the introduction of the drug. Learning how to manage and prevent resistance is a major medical challenge that requires an understanding of the evolutionary dynamics of each pathogen. This Review summarizes the similarities and differences in the evolution of drug resistance for these three pathogens.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Ahmad Z, Makaya NH, Grosset J. In: Antituberculosis Chemotherapy. Donald PR, Helden PD, Bolliger CT, Karger SAG, editors. Vol. 40. 2011. pp. 1–8.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

